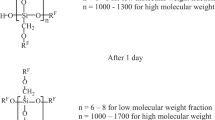Abstract
It has been established that radical polymerization of tetrafluoroethylene (TFE) in liquid alkoxysilanes (dimethyldimethoxy, trimethoxy, methyltrimethoxy, or tetramethoxysilane) yields fluorinated organosilicon oligomers with the general formula R(C2F4)nH, where RH is an alkoxysilane molecule capable of further polycondensation. The composition of the terminal groups and the molecular-mass distribution of the oligomers have been determined from mass spectra, NMR spectra, and differential thermogravimetric curves. With increasing TFE concentration in solutions, the chain growth is accompanied by a transition from a homogeneous to a colloidal solution and then to a gel of oligomers. At maximum TFE concentrations, the chain length n in colloidal solutions reaches 10–12. The colloidal particles are composed of oligomers and solvent molecules, the number of which per one monomer unit is minimal in the gel to be 4–6. On the basis of the oligomers obtained, it is feasible to create coatings having both high hydrophobicity and strong bonding with the protected surface.





Similar content being viewed by others
REFERENCES
Quere, D., Rep. Progr. Phys., 2005, vol. 68, p. 2495.
Boinovich, L.B. and Emel’yanenko, A.M., Usp. Khim., 2008, vol. 77, p. 619.
Sarkar, D.K. and Farzaneh, M. J. Adhes. Sci. Technol., 2009, vol. 23, no. 9, p. 1215.
Nakajima, A., NPG Asia Mater., 2011, vol. 3, p. 49.
Yan, Y.Y., Gao, N., and Barthlott, W., Adv. Colloid Interface Sci., 2011, vol. 169, p. 80.
Kim, I.P. and Shestakov, A.F., High Energy Chem., 2009, vol. 43, no. 6, p. 460.
Kim, I.P., Martynenko, V.M., Shul’ga, Yu.M., and Shestakov, A.F., High Energy Chem., 2010, vol. 44, no. 6, p. 449.
Kim, I.P., Martynenko, V.M., Chernyak, A.V., and Benderskii, V.A., High Energy Chem., 2017, vol. 51, no. 4, p. 285.
Ruppert, I., Schlich, K., and Volbach, W., Tetrahedron Lett., 1984, vol. 25, p. P. 2195.
Petrov, V.A., Tetrahedron Lett., 2001, vol. 42, p. 3267.
Boyko, V.E., Tyutyunov, A.A., Don, V.I., and Igoumnov, S.M., Fluorine Notes, 2013, vol. 6, p. 91.
Kulinich, S.A., Farhadi, S., Nose, K., and Du, X.M., Langmuir, 2011, vol. 27, p. 25.
Marby, J.M., Vij, A., Iacono, S.T., and Viers, B.D., Angew. Chem., Int. Ed. Engl., 2008, vol. 47, p. 4137.
Comprehensive Chemical Kinetics, vol. 14A: Free Radical Polymerization, Bamford, C.H. and Tipper, C.F.H., Eds., Amsterdam: Elsevier, 1976.
Kim, I.P. and Benderskii, V.A., High Energy Chem., 2011, vol. 45, no. 5, p. 372.
Kim, I.P., Kunitsa, A.A., and Chernyak, A.V., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 11, p. 1845.
Kim, I.P., Izv. Akad. Nauk, Ser. Khim., 2013, vol. 62, no. 9, p. 2065.
Kichigina, G.A., Kushch, P.P., Bolshakov, A.I., Kiryukhin, D.P., and Buznik, V.M., Khim. Vys. Energ., 2012, vol. 46, no. 3, p. 143.
Kim, I.P. and Benderskii, V.A., Russ. J. Phys. Chem. A, 2014, vol. 88, no. 11, p. 1954.
Stein, S.E. and Brown, R.L., J. Chem. Inform. Comput. Sci., 1994, vol. 34, p. 581.
Kim, I.P., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 7, p. 1079.
Kim, I.P. and Kolesnikova, A.M., Russ. J. Phys. Chem. A, 2011, vol. 85, no. 9, p. 1660.
Kim, I.P. and Benderskii, V.A., High Energy Chem., 2015, vol. 49, no. 1, p. 1.
Laikov, D.N., J. Chem. Phys., 2011, vol. 135, p. 134120.
Yilmazer, N.D. and Korth, M., Comp. Struct. Biotechnol. J., 2015, vol. 13, p. 169.
Kim, I.P., Kunitsa, A.A., and Chernyak, A.V., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 11, p. 1845.
Ameduri, B., Boutevin, B., Well-architectured fluoropolymers: synthesis, properties and applications, Amsterdam. Elsevier, 2004.
Kim, I.P., Martynenko, V.M., Chernyak, A.V., and Benderskii, V.A., High Energy Chem., 2017, vol. 51, no. 4, p. 277.
Dalvi, V.H. and Rossky, P.J., Proc. Natl. Acad. Sci. USA, 2010, vol. 107, p. 13603.
Tsige, M., Curro, J.G., and Grest, G.S., J. Chem. Phys., 2008, vol. 129, p. 214901.
Tsige, M. and Grest, G.S., J. Phys. Chem. C, 2008, vol. 112, p. 5029.
ACKNOWLEDGMENTS
The work was supported by the Presidium of the Russian Academy of Sciences under Basic Research Program PFI I.8P and performed under state task no. 0089-2014-0025.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Kim, I.P., Martynenko, V.M., Chernyak, A.V. et al. Fluorinated Organosilicon Oligomers with End Groups Capable of Further Polycondensation. High Energy Chem 53, 95–102 (2019). https://doi.org/10.1134/S0018143919020073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143919020073