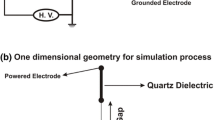
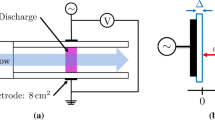
Abstract
A method is proposed for the simulation of chemical kinetics in a dielectric barrier discharge (DBD) with the use of an effective rate constant of electron–molecule reaction and a simple expression for its estimation. The results of the simulation of the kinetics of methane conversion in DBD in the presence of water are presented.
Similar content being viewed by others
References
Arutyunov, V.S., Okislitel’naya konversiya prirodnogo gaza (Oxidative Conversion of Natural Gas), Moscow: Krasand, 2011.
Kudryashov, S.V., Ryabov, A.Yu., and Ochered’ko, A.N., High Energy Chem., 2017, vol. 51, no. 2, p.128.
Samoilovich, V.G., Gibalov, V.I., and Kozlov, K.V., Fizicheskaya khimiya bar’ernogo razryada (Physcial Chemistry of Dielectric Barrier Discharge), Moscow: Izd. MGU, 1989.
Indarto, A., Coowanitwong, N., Choi, J., Lee, H., and Song, H.K., Fuel Process. Technol., 2008, vol. 89, no. 2, p.214.
Nair, S.A., Nozaki, T., and Okazaki, K., Chem. Eng. J., 2007, vol. 132, nos. 1–3, p.85.
Ravasio, S. and Cavallotti, C., Chem. Eng. Sci., 2012, vol. 84, p.580.
Lovascio, S., Blin-Simiand, N., Magne, L., Jorand, F., and Pasquiers, S., Plasma Chem. Plasma Process., 2015, vol. 35, no. 2, p.279.
Goujard, V., Tatibouet, J.-M., and Batiot-Dupeyrat, C., Plasma Chem. Plasma Process., 2011, vol. 31, no. 2, p.315.
Kovács, T., Plasma Chem. Plasma Process., 2009, vol. 30, no. 1, p.207.
Istadi, I. and Amin, N.A.S., Chem. Eng. Sci., 2007, vol. 62, no. 23, p. 6568.
Non-Thermal Plasma Techniques fro Pollution Control, Penetrante, B.M. and Schultheis, S.E., Eds., Berlin: Springer, 1993.
Hagelaar, G.J.M. and Pitchford, L.C., Plasma Sources Sci. Technol., 2005, vol. 14, no. 4, p.722.
Viehland database. http://www.lxcat.net.
Janev, R.K. and Reiter, D., Phys. Plasmas, 2002, vol. 9, no. 9, p. 4071.
Ianni, J.C., Kintecus v. 5.5. 2015. http://www.kintecus. com.
NIST Chemical Kinetics Database. http://kinetics. nist.gov.
Ianni, J.C., Atropos v.1. 2003. http://www.kintecus. com/atropos.htm.
Baulch, D.L., Cobos, C.J., Cox, R.A., Esser, C., Frank, P., Just, Th., Kerr, J.A., Pilling, M.J., Troe, J., Walker, R.W., and Warnatz, J., J. Phys. Chem. Ref. Data, 1992, vol. 21, no. 3, p.411.
Braun, W., J. Chem. Phys., 1967, vol. 46, no. 6, p. 2071.
Braun, W., J. Chem. Phys., 1970, vol. 52, no. 10, p. 5131.
Halberstadt, M.L. and Crump, J., J. Photochem., 1972, vol. 1, no. 4, p.295.
Galland, N., Caralp, F., Hannachi, Y., Bergeat, A., and Loison, J.-C., J. Phys. Chem. A, 2003, vol. 107, no. 28, p. 5419.
Tsang, W. and Hampson, R.F., Chem. Ref. Data, 1986, vol. 15, no. 3, p. 1087.
Curran, H.J., Int. J. Chem. Kinet., 2006, vol. 38, no. 4, p.250.
Tsang, W., J. Phys. Chem. Ref. Data, 1988, vol. 17, no. 2, p.887.
Zabarnick, S., Fleming, J.W., and Lin, M.C., Symp. Combust., 1988, vol. 21, no. 1, p.713.
Jasper, A.W., Klippenstein, S.J., Harding, L.B., Ruscic, B., and Jasper, A.W., J. Phys. Chem. A, 2007, vol. 111, no. 19, p. 3932.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.V. Kudryashov, A.Yu. Ryabov, A.N. Ochered’ko, 2018, published in Khimiya Vysokikh Energii, 2018, Vol. 52, No. 2, pp. 150–153.
Rights and permissions
About this article
Cite this article
Kudryashov, S.V., Ryabov, A.Y. & Ochered’ko, A.N. Simulation of the Kinetics of Methane Conversion in the Presence of Water in a Barrier Discharge. High Energy Chem 52, 167–170 (2018). https://doi.org/10.1134/S0018143918020108
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143918020108