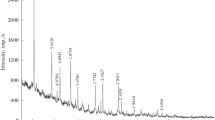
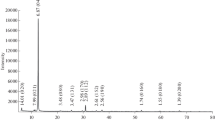
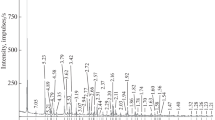
Abstract—
Two sodalite samples (sample I is Na8Al6Si6O24Cl2⋅0.4H2O from the Kovdor alkaline ultramafic massif with carbonatites in the Murmansk region, Russia, and sample II is Na8Al6Si6O24Cl2⋅0.2H2O from the Bayan Kol nepheline syenite and miaskite massif in the Republic of Tyva) were studied by thermal and electron-microprobe analyses, powder X-ray diffraction, photoluminescence, and by IR, Raman, and ESR spectroscopy. Solution melt calorimetry was applied to determine the enthalpy of formation from elements for water-bearing sodalite samples: −13536 ± 10 (I) and −13503 ± 19 (II) kJ/mol. The enthalpy of formation of sodalite of the theoretical composition Na8Al6Si6O24Cl2 was evaluated at ΔfH0(298.15 K) = −13446 ± 11 kJ/mol. The data obtained on the enthalpy of formation of sodalite and literature data on its S0(298.15 K) were used to calculate the standard Gibbs energies of formation of anhydrous and of water-bearing sodalite.





Similar content being viewed by others
REFERENCES
E. D. Andreeva, V. A. Kononova, E. V. Sveshnikova, and R. M. Yashina, Igneous Rocks. Volume 2. Alkaline Rocks (Nauka, Moscow, 1984) [in Russian].
H. Annersten and A. Hassib, “Blue sodalite,” Can. Mineral. 17, 39–46 (1979).
S. Antao and I. Hassan, “Thermal analyses of sodalite, tugtupite, danalite and helvite,” Can. Mineral. 40, 163–172 (2002).
M. C. Barnes, J. Addai-Mensah, and A. R. Gerson, “A methodology for quantifying sodalite and cancrinite phase mixtures and the kinetics of the sodalite to cancrinite phase transformation,” Micropor. Mesopor. Mater. 31, 303–319 (1999).
B. E. Borutskii, Rock-Forming Minerals of High-Alkali Complexes (Nauka, Moscow, 1988) [in Russian].
N. F. Cano, A. R. Blak, and S. Watanabe, “Correlation between electron paramagnetic resonance and thermoluminescence in natural sodalite,” Phys. Chem. Miner. 37, 57–64 (2010).
N. F. Cano, A. R. Blak, J. S. Ayala-Arenas, and S. Watanabe, “Mechanisms of TL for production of the 230°C peak in natural sodalite,” J. Lumin. 131, 165–168 (2011).
N. V. Chukanov, M. F. Vigasina, N. V. Zubkova, I. V. Pekov, C. Schäfer, A. V. Kasatkin, V. O. Yapaskurt, and D. Yu. Pushcharovsky, “Extra-framework content in sodalite–group minerals: complexity and new aspects of its study using infrared and Raman spectroscopy,” Mineralogy 10, # 363 (2020).
N. V. Chukanov, R. Yu. Shendrik, M. F. Vigasina, I. V. Pekov, A. N. Sapozhnikov, V. D. Shcherbakov, and D. A. Varlamov, “Crystal chemistry, isomorphism, and thermal conversions of extra-framework components in sodalite–group minerals,” Minerals 12, # 887 (2022a).
N. V. Chukanov, N. V. Zubkova, I. V. Pekov, R. Yu. Shendrik, D. A. Varlamov, M. F. Vigasina, D. I. Belakovskiy, S. N. Britvin, V. O. Yapaskurt, and D. Yu. Pushcharovsky, “Sapozhnikovite, Na8(Al6Si6O24)(HS)2, a new sodalite-group mineral from the Lovozero alkaline massif, Kola Peninsula,” Mineral. Mag. 86, 49–59 (2022b).
R. A. Denisov, V. P. Denks, A. E. Dudelzak, V. S. Osminin, and T. V. Ruus, “Optically erasable coloration and luminescence of sodalites,” Zh. Phys. Chem. 27 (1), 149–154 (1977).
M. Dumańska-Słowik, W. Heflik, A. Pieczka, and M. Sikorska, “The transformation of nepheline and albite into sodalite in pegmatitic mariupolite of Oktiabrski Massif (SE Ukraine),” Spectrachim. Acta. Part A: Mol. Biomol. Spectr. 150, 837–845 (2015).
C. Günther, H. Richter, I. Voigt, A. Michaelis, H. Tzscheutschler, R. Krause-Rehberg, and J. M. Serra, “Synthesis and characterization of a sulfur containing hydroxyl sodalite without sulfur radicals,” Micropor. Mesopor Mater. 214, 1–7 (2015).
A. Hassib, O. Beckman, and H. Annersten, “Photochromic properties of natural sodalite,” J. Phys. D: Appl. Phys. 10, 771–777 (1977).
K. Hettmann, T. Wenzel, M. Marks, and G. Markl, “The sulfur speciation in S-bearing minerals: New constraints by combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals,” Am Mineral. 97, 1653–1661 (2012).
W. G. Hodgson, J. S. Brinen, and E. F. Williams, “Electron spin resonance investigation of photochromic sodalities,” J. Chem. Physics. 47 (10), 3719–3723 (1967).
G. Yu. Ivanyuk and V. N. Yakovenchuk, Minerals of Kovdor (Kolskii NTs RAN, Apatity, 1997) [in Russian].
I. A. Kiseleva, L. P. Ogorodova, N. D. Topor, and O. G. Chigareva, “Thermochemical study of the CaO–MgO–SiO2 system,” Geokhimiya, no. 12, 1811–1825 (1979).
I. A. Kiseleva, L. P. Ogorodova, Yu. I. Sidorov, and I. L. Khodakovskii, “Thermodynamic properties of alkaline feldspars,” Geokhimiya, No. 3, 406–413 (1990).
I. A. Kiseleva, A. Navrotsky, I. A. Belitsky, and B. A. Fursenko, “Thermochemical study of calcium zeolites—heulandite and stilbite,” Am. Mineral. 86, 448–455 (2001).
L. N. Kogarko, Problems of Genesis of Agpaitic Magmas (Nauka, Moscow, 1977) [in Russian].
N. Komada, E. F. Westrum, B. S. Hemingway, M. Yu. Zolotov, Yu. V. Semenov, I. L. Khodakovsky, and L. M. Anovitz, “Thermodynamic properties of sodalite at temperatures from 15 K to 1000 K,” J. Chem. Thermodynam. 27, 1119–1132 (1995).
A. R. Kotelnikov, L. V. Zhornyak, and Z. A. Kotel’nikova, “Distribution of sulfur between sodalite and hydrothermal fluid: an experimental study,” Geochem. Int. 34 (11), 975–979 (1996).
S. Lin, M. Wang, Ya. Hao, K. Zhang, Yu. Li, and D. Yang, “Synthesis, structure and thermal stability of iodine–contained sodalities Na8(AlSiO4)6Cl2 – xIx (x = 0–2) for 129I immobilization,” J. Alloys Compd. 908, # 164617 (2022).
S. D. McLaughlan and D. J. Marshall, “Paramagnetic resonance of F-type centers in photochromic sodalities,” Phys.Lett. 32A, 343–344 (1970).
G. B. Naumov, B. N. Ryzhenko, and I. L. Khodakovskii, Reference Book of Thermodynamic Values (for Geologists) (Atomzidat, Moscow, 1971) [in Russian].
I. Norrbo, P. Gluchowski, P. Paturi, J. Sinkkonen, and M. Lastusaari, “Persistent luminescence of tenebrescent Na8Al6Si6O24(Cl,S)2,” Inorg. Chem. 54, 7717–7724 (2015).
L. P. Ogorodova, L. V. Melchakova, I. A. Kiseleva, and I. A. Belitsky, “Thermochemical study of natural pollucite,” Thermochim. Acta. 403, 251–256 (2003).
L. P. Ogorodova, I. A. Kiseleva, L. V. Melchakova, M. F. Vigasina, and E. M. Spiridonov, “Enthalpy of formation of talc Mg3[Si4O10](OH)2 according to dissolution calorimetry,” Russ. J. Phys. Chem. 85 (9), 1489–1491 (2011).
R. C. Peterson, “The structure of hackmanite, a variety of sodalite, from Mont St-Hilaire, Quebec,” Can.Mineral. 21, 549–552 (1983).
P. S. Pizani, M. C. Terrile, H. A. Farach, and C. R. Poole, “Color centers in sodalite,” Am. Mineral. 70, 1186–1192 (1985).
V. Yu. Prokof’ev and N. E. Gordina, “Preparation of granulated LTA and SOD zeolites from mechanically activated mixtures of metakaolin and sodium hydroxide,” Appl. Clay Sci. 101, 44–51 (2014).
T. A. Radomskaya, E. V. Kaneva, R. Yu. Shendrik, L. F. Suvorova, and N. V. Vladykin, “Sulfur-bearing sodalite–hackmanite in the alkaline pegmatites of the Inagli massif (Aldan shield): crystal chemical features, photochromism, and luminescence,” Zap. Ross. Mineral. O-va 149 (2), 42–54 (2020).
R. A. Robie and B. S. Hemingway, “Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures,” U S Geol. Surv. Bull., No. 2131, (1995).
A. A. Rogozhin, B. S. Gorobets, and S. V. Ryabenko, “Nature of luminescence of haloid and haloid-bearing minerals,” Mineral. Zh. 4 (2), 45–52 (1982).
Z. D. Sharp, G. R. Helffrich, S. R. Bohlen, and E. J. Essene, “The stability sodalite in the system NaAlSiO4–NaCl,” Geochim. Cosmochim Acta 53, 1943–1954 (1989).
A. Škvarlová, M. Kanuchová, L. Kozáková, E. Valušová, and M. Holub, “Preparation and characterization of ultramarine blue pigments from fly ash by using the X-ray photoelectron spectroscopy (XPS) for the determination of chemical states of sulphur in chromophores,” Micropor. Mesopor. Mater. 284, 283–288 (2019).
A. N. Tarashchan, Luminescence of Minerals (Naukova dumka, Kiev, 1978) [in Russian].
A. N. Tarashchan, A. N. Platonov, L. V. Bershov, and V. P. Belichenko, Constitution and Properties of Minerals (Naukova dumka, Kiev, 1970), Vol. 4, pp. 63–65 [in Russian].
M. J. Taylor, D. J. Marshall, and H. Evans, “Infra-red spectra of photochromic sodalities,” J. Phys. Chem. Solids. 32, 2021–2026 (1971).
E. R. Vance, D. J. Gregg, I. Karatchevtseva, J. Davis, and M. Ionescu, “He and Au ion radiation damage in sodalite, Na4Al3Si3O12Cl,” J. Nucl. Mater. 453, 307–312 (2014).
S. C. Zilio and V. S. Bagnato, “Infrared spectra of natural sodalite,” J. Chem. Phys. 88, 1373–1376 (1984).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Kurdyukov
Rights and permissions
About this article
Cite this article
Gritsenko, Y.D., Eremina, E.N., Vigasina, M.F. et al. Sodalite: Spectroscopic and Thermochemical Investigations. Geochem. Int. 61, 735–743 (2023). https://doi.org/10.1134/S0016702923060046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702923060046