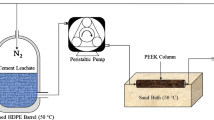
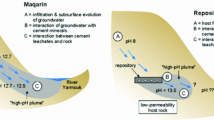
Abstract
In Gulu area, geothermal waters show a high concentration of alkali metals (Li, Rb and Cs) and some other typical pollution elements, e.g. As, Sb and Tl. They are also enriched in the geothermal deposits during the phase separation of silica sinter and residual geothermal water. A comprehensive sequential extraction was carried out on the Gulu silica sinter. The results indicated that significant Li and K amount is bound to the Fe-Mn oxide and the residual fractions of sinter while, the elements (B, Cs, Rb, As, Sb and Tl) are predominantly concentrated in the sinter crystal lattices. In addition, the elemental distribution law during phase separation of the residual geothermal water and associated evapoconcentrated deposits is also uncovered in this study. With the respect to the system of the natural silica sinters precipitated from the mother geothermal water, complex variations occur during the trace elements partition between the mother water and associated deposits under evapoconcentration. Thus, the miscellaneous minerals (Li, Rb, K, Cs and B) are also distributed in the solid phases when special minerals such as opal, borax or sylvite precipitate from the residual fluids. This confirms that the valuable minerals of K, B, Cs, Rb or Li can precipitate when the geothermal water is evapoconcentrated to a certain degree and within a very concentrative stage of density. However, these elements are remarkably depleted in the residual geothermal fluid at the stages corresponding to the precipitation of these minerals while they are highly enriched in the mother fluid during the late stages of the evapoconcentration. Thus, it can be stated for the first time that the high concentration of these elements in the residual waters at the final stages will be certainly used in the future. In contrast, the concentration of pollution elements (As, Sb and Tl) of the geothermal solids is higher in the early stage of the opal precipitation, while it is lower in the later stages. The concentration of these elements (As, Sb and Tl) in residual geothermal fluids distinctly increases and is very high at the final stages of the evapoconcentration. This scenario indicates that large quantities of toxic elements such as As, Tl and Sb may degrade the surface and ground waters resources located at or near the geothermal water by mixing process making them unsuitable for drinking or irrigation. Thus, in addition to the thermal energy provided, the geothermal area are very important prospect for exploration and extraction of the mineral resources of the elements such as K, Li, B, Rb or Cs that have very significant economic value. However, percentages of trace elements such as Li, Tl, Rb and K in the exchangeable and carbonates fractions of this sinter suggested that they could become rapidly bioavailable in the geothermal environment.




Similar content being viewed by others
REFERENCES
R. Armijo, P. Tapponnier, J. L. Mercier, and T. L. Han, “Quaternary extension in southern Tibet: field observation and tectonic implications,” J. Geophys. Res. 91, 13803–138772 (1986).
N. Belzile and A. Tessier, “Interaction between arsenic and iron oxyhydroxides in lacustrine sediments,” Geochim. Cosmochim. Acta 54, 103–109 (1990).
K. I. Brown and S. F. Simmon, “Precious metals in high–temperature geothermal systems in New Zealand,” Geothermics 32, 619–625 (2003).
A. V. Corrona, N. Vigiera, R. Millot, and B. S. Hardarson, “Lithium isotopes in hydrothermal altered basalts from Hengill (SW Iceland),” Earth Planet. Sci. Lett. 411, 62–71 (2015)
L. Ding, D. Zhong, A. Yin, P. Kapp, T. M. Harrison, “Cenozoic structural and metamorphic evolution of the eastern Himalayan syntax (Namche Barwa),” Earth Planet Sci. Lett. 192 (3), 423–438 (2001).
Grimaud, D. S. Huang, G. Michard, and K. Zheng, “Chemical study of geothermal waters of central Tibet (China),” Geothermics 14, 35–48 (1958).
S. Gatehouse, D. W. Russell, J. C. Van Moort, “Sequential soil analysis in exploration geochemistry,” J. Geochem. Explor. 8, 483–498 (1977).
C. Gleyzes, S. Tellier, and M. Astruc, “Fractionation studies of the trace elements in contaminated soils and sediments: a review of the sequential extraction procedures,” Trends Analyt. Chem. 21, 451–467 (2002).
Q. Guo, Y. Wang, and W. Liu, “Major hydrochemical processes in two reservoirs of the Yangbajing geothermal field, China,” J. Volcanol. Geotherm. Res. 166, 255–268 (2007).
T. L. Han, Evolution on the Lithospheric Deformation of the Himalayan: Tibetan Active Tectonics (Geological Publishing House, 1987), pp. 35–41 (in Chinese).
G. E. M. Hall, G. Gauthier, J. C. Pelchat, P. Pelchat, J. E. Vaive, “Application of a sequential extraction scheme to ten geological certified reference materials for the determination of 20 elements,” Can. J. Analyt. Atom. Spectrom. 11 (9), 787–796 (1996).
Z. Liao and P. Zhao, Yunnan–Tibet Geothermal Belt: Geothermal Resources and Case Histories (Science Press, Beijing, 1999).
Z. Q. Li, Z. Q. Hou, F. J. Nie, Z. S. Yang, X. M. Qu, X. J. Meng, and Y. Y. Zhao, “Enrichment of element cesium during modern geothermal action in Tibet,” China. Acta Geol. Sin. 80, 1457–1464 (2006) [in Chinese with English abstract].
J. T. Landrum, P. C. Bennett, A. S. Engel, M. A. Alsina, P. A. Pasten, and K. Milliken, “Partitioning geochemistry of arsenic and antimony, El Tatio geyser field, Chile,” Appl. Geochem. 24, 664–676 (2009).
A. Milorad, “Cesium and other alkali elements in thermal springs of Gornja Trepca and Mlakovak thermal springs,” Rad. Inst. Geol.–Rud. Instraz Inspit. Nukl. Drugih. Miner. Sirovina 6, 243–247 1960.
A. Milorad and T. Milorad, Geochemical and geological features of the thermal springs of Gornja–Trepca. Geol.–Rud. Instraz Inspit. Nukl. Drugih. Miner. Sirovina 6, 235–242 (1969).
J. E. McKenzie, K. L. Brown, S. L. Cady, and K. A. Campbell, “Trace metal chemistry and silicification of microorganisms in geothermal sinter, Taupo Volcanic Zone, New Zealand,” Geothermics. 30 (2001) 483–502 (2001).
M. O. Muñoz, P. Bhattacharya, O. Sracek, O. R. Ramos, J. Q. Aguirre, J. Bundschuh, and J. P. Maity, “Arsenic and other trace elements in the thermal springs and in cold waters from drinking wells on the Bolivian Altiplano,” J. S. Am. Earth Sci. 60, 10–20 (2015).
D. Mendrinos, I. Choropanitis, O. Polyzou, and C. Karystsas, “Exploring for geothermal resources in Greece,” Geothermics. 39, 124–137 (2019).
R. J. Parker and K. Nicholson, “Arsenic in geothermal sinters: determination and for mineral exploration,” 12 th NZ. Geotherm. Work (1990), pp. 35–39.
A. Pentecost, “Geochemistry of carbon–dioxide in travertine-depositing waters of Italy,” J. Hydrol. 167, 263–278 (1998).
L. Romero, H. Alonso, P. Campano, I. Fanfani, R. Gidu, C. Dadea, T. Keegan, I. Thornton, and M. Farago, “Arsenic enrichment in waters and sediment of Rio Loa (Second region, Chile),” Appl. Geochem. 18 (9), 1339–1416 (2003).
J. E. Sanders and G. M. Friedman, “Origin and occurrence of limestones,” In: Carbonate Rocks, Development in Sedimentology, Ed. by G. V. Chilinger, H. J. Bissell, and R. W. Fairbridge (Elsevier, Amsterdam, 1967), vol. 9.
E. R. Stauffer and J. M. Thompson, “Arsenic and antimony in geothermal water of Yellowstone National Park, Wyoming, USA,” Geochim. Cosmochim. Aata 48, 2547–2561 (1984).
H. Saidi and S. Ehara, “Temperature and chemical changes in the fluids of the Obama geothermal field (SW Japan) in response to field utilization,” Geothermics 39 (3), 228–241 (2010).
A. Tessier, P. G. C. Campbell, and M. Bisson, “Sequential extraction procedure for speciation of trace metals,” Anal. Chem. 51, 844–851 (1979).
P. Tapponnier, J. L. Mercier, R. Armijo, H. Tonglin, and Z. Ji, “Field evidence for active normal faulting in Tibet,” Nature 294, 410–414 (1981).
P. Tapponnier, Z. Q. Xu, F. Roger, B. Meyer, N. Arnaud, G. Wittlinger, and J. S. Yang, “Oblique stepwise rise and growth of the Tibet plateau,” Science 294, 1671–1677 (2001).
W. Tong, S. B. Liao, Z. F. Zhang, M. Z. You, and M. T. Zhang, Thermal Springs in Tibet (Science Press, Beijing, 2000)
X. Tang, J. Zhang, Z. Pang, S. Hu, Y. Wu, and S. Bao, “Distribution and genesis of the eastern Tibetan Plateau geothermal belt, western China,” Environ. Earth Sci.76 (1), 31 2017.
H. Tan, P. Su, T. Don, and I. E. Hartman, “Enrichment mechanism of Li, B and K in the geothermal water and associated deposits from the Kawu area of the Tibetan plateau: constraints from geochemical experimental data,” Appl. Geochem. 93 (2018) 60–68 (2018).
R. E. Villanueva–Estrada, R. M. Prol–Ledesma, A. Rodríguez–Díaz, C. Canet, and M. A. Armienta, “Arsenic in hot springs of Bahía Concepción, Baja California Peninsula, México,” Chem. Geol. 348 (2013), 27–36 (2012).
Word Health Organization. Guidelines for Drinking-Water Quality, 2nd Ed. (WHO, Geneva, 1996).
Yong, R. N. R. Galvarez–Cloutier, and Y. Phadungchewit, “Selective sequential extraction analysis of heavy–metal retention in soil,” Can. Geotech. J. 30, 834–847 (1993).
A. Yin, and T. M. Harrison, “Geologic evolution of the Himalayan–Tibetan orogeny,” Annu. Rev. Earth Planet. Sci. 28, 211–280 (2000).
Z.-J. Yao, X.-G. Zhang, K.-S. An, and Z.-H. Zheng, “An assessment of geological resources in Yangbajing, Xizang (Tibet),” Bull. Inst. Hydrogeol. Eng. Geol. CAGS 2, 1–51 1986 [In Chinese, with English Abstr.].
S. C. Yanez, M. Reich, M. Leisen, D. Morata, and F. Barra, “Geochemistry of metals and metalloids in siliceous sinter deposits: Implications for elemental partitioning into silica phases,” Appl. Geochem. 80 (2017), 112–133 2017.
K. J. Zhang, Y. X. Zhang, X. C. Tang, and B. Xia, “Late Mesozoic tectonic evolution and growth of the Tibetan plateau prior to the Indo–Asian collision,” Earth Sci. Rev. 114 (3–4), 236–249 (2012).
W. Zhang, H. Tan, Y. Zhang, W. Wei, and T. Dong, “Boron geochemistry from some typical Tibetan hydrothermal systems: origin and isotopic fractionation,” Appl. Geochem. 63, 436–445 (2015).
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (41872074, 91747203) and the Fundamental Research Funds for the Central Universities (2017B19614).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartman Issombo Elenga, Tan, H., Shi, D. et al. Elemental Distribution and Partitioning Law between the Geothermal Water and Associated Deposits for a Typical Geothermal System with Large-Scale Siliceous Sinter Deposits in the Tibet. Geochem. Int. 59, 1258–1273 (2021). https://doi.org/10.1134/S0016702921100037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702921100037