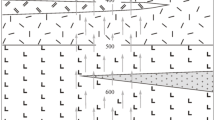
Abstract—The base-metal (70–75 wt %) and tin–sulfide (10–15 wt %) deposits are the main indium suppliers in the world. However, the causes and conditions of In accumulation in the ores of these deposits are still unclear. To shed light on this problem, we simulated the physicochemical conditions of formation of base metal and tin–sulfide ores with elevated indium content. For this purpose, the average composition of ore–bearing hydrothermal solutions and parameters of ore precipitation at these deposits were determined using available literature data. Based on these data, obtained standard thermodynamic characteristics \(\Delta G_{{\text{f}}}^{0},\)\(\Delta H_{f}^{0},\)\(S_{f}^{0},\)\(V_{f}^{0},\)\(C_{p}^{0}\) of chloride indium species (\({\text{InCl}}_{2}^{ + },\) InCl3, InClOH+), coefficients required for calculations at elevated temperature and pressure, the formation of elevated indium contents in these ores was numerically simulated using “Gem–Selektor-3” and “Chiller” softwares. The results of thermodynamic modeling of the formation of quartz–cassiterite and tin–sulfide ores show that the higher In contents in tin–sulfide ores are related to their formation from acid (pH 4.3), high–chloride (6.6 m) solutions, which contain In (0.002 m) in form of (InCl3aq). The quartz–cassiterite ores were formed from near–neutral (pH 5.3), low–chloride (1.02 m) solution, in which In occurred as hydroxocomplexes InO2H and \({\text{InO}}_{2}^{ - }\) in concentrations no more than 0.00004 m, which, respectively, determined its low contents in these ores. Computer modeling of the formation of indium–bearing sulfide-base metal and barite-base metal deposits shows that they were formed form high–temperature chloride (1.3–4.3 m) hydrothermal solutions of near–neutral composition (pH 5.8–6). The main In speciations are hydroxocomplexes InO2H and \({\text{InO}}_{2}^{ - }\), which provide In concentrations of 5–9 × 10–5 m). However, due to the low indium concentrations in hydrothermal solutions, the forming sulfide minerals (sphalerite, pyrite, and chalcopyrite) differ in the lower indium contents compared to the minerals of tin–sulfide ores.


Similar content being viewed by others
REFERENCES
V. I. Altynov, and B. V. Ptitsyn, “Theory of chlorine–silver electrode and determination of instability constants of complex chlorides,” Zh. Inorg. Khim. 7 (9), 2103–2109 (1962).
A. D. Audetat, Gunther, and C. A. Heinrich, “Magmatic–hydrothermal evolution in fractionating granite: a microchemical study of the Sn–W–F-mineralized Mole Granite (Australia),” Geochim. Cosmochim. Acta 64, 3373–3393 (2000).
C. E. Baes Jr. and R. E. Mesmer, The Hydrolysis of Cations (Krieger Publishing Company, Malabar, 1986).
V. I. Belevantsev, G. R. Kolonin, and B. I. Peshchevetskii, “On the ways of estimating the stability constant and the possible role in the mineral formation of mixed complex compounds: evidence from hydroxohalogenocomplexes,” The Main Parameters of Natural Processes of Endogenous Ore Formation (SO AN SSSR, Novosibirsk, 1977), Vol. 3, pp. 62–63 [in Russian].
V. I. Belevantsev, L. V. Gushchina, and A. A. Obolensky, “Hydrothermal solutions and mercury migration,” Hydrothermal Low-Temperature Ore-Formation and Metasomatism (Nauka, Novosibirsk, 1982), pp. 3–49 [in Russian].
M. Benzaazoua, P. Marion, A. Pinto, H. Migeon, and F. E. Wagner, Mineral. Engineer. 16, 1291–1302 (2003).
A. S. Borisenko, A. I. Kholmogorova, A. A. Borovikov, A.P. Shebanin, and V. V. Babich, “Composition and metal potential of ore-forming solutions of the Deputatskoe tin deposit, Yakutia,” Geol. Geofiz. 38 (11), 1830–1841 (1997).
A. S. Borisenko, G. G. Pavlova, A. A. Borovikov, and N. V.Bryanskii, “Composition and mtal potential of ore-forming fluids of the Deputatskoe Sn–W (Ag) deposit, Yakutia,“ Proceed. Conference (Inst. Geokhim. SO RAN, Irkutsk, 2014), pp. 8–9 [in Russian].
N. S. Bortnikov, V. A. Simonov, E. E. Amplieva, and A. A. Borovikov, “Anomalously high concentrations of metals in fluid of the Semenov modern hydrothermal system (Mid-Atlantic Ridge, 1331 N): LA–ICP–MS study of fluid inclusions in minerals,” Dokl. Earth Sci. 456 (2), 714–719 (2014).
A. I. Busev and N. A. Kanaev “Calculation of stability constants of some complex indium compounds by a method of constant difference: data obtained by cationites,” Vestn. Mosk. Univ. Ser. Matematika, Mekhanika, Astronomiya, Fizika, Khimiya, 1, 135–143 (1959).
M. N. Butova, L. B. Zubkov, and L. B. Chistov, “Problems of the development of raw base and indium production. Mineral resources of Russia,” Ekonomika Upravlenie 4, 3–8 (1998).
B. G. F. Carleson and H. Irving, “The stability constants of the indium halides,” J. Chem. Soc. 4390–4399 (1954).
J. Cauzid, P. Philippot, G. Martinez-Criado, B. Menez, and S. Laboure, “Contrasting Cu–complexing behavior in vapor and liquid fluid inclusions from Ynkee Lode tin deposit, Mole Granite, Australia,” Chem. Geol. 246, 39–54 (2007).
C. V. Churakov, C. I. Tkachenko, M. A. Korzhinsky, R. E. Botcharnikov, and K. I. Shmulovich, “Evolution of composition of high–temperature fumarolic gases from Kudryavy volcano, Iturup, Kuril Islands: the thermodynamic modeling,” Geochem. Int. 38(5), 436–451 (2000).
C. E. J. de Ronde, G. J. Massoth, D. A. Butterfield, B. W. Christenson, J. Ishibashi, R. G. Ditchburn, M. D. Hannington, R. L. Brathwaite, J. E. Lupton, V. S. Kamenetsky, I. J. Graham, G. F. Zelmer, R. P. Dziak, R. W. Emley, V. M. Dekov, F. Munnik, J. Lahr, L. J. Evans, and K. Takai, “Submarine hydrothermal activity and gold–rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand,” Mineral. Deposita 46, 541–584 (2011).
P. Debye and E. Huckel, “Zur theorie der Electrolyte,” Phys. Zeitsch., Bd 24 (9), 185–206 (1923).
D. Ferri, “On the complex formation equilibria between indium(III) and chloride ions,” Acta Chem. Scand. 26, 733–746 (1972a).
D. Ferri, “On the hydrolysis of the indium(III) ion in chloride solutions,” Acta Chem. Scand. 26, 747–759 (1972b).
I. V. Gaskov, N. Yu. Mironov, and A. P. Pertsev, “Geochemistry of sulfide–base metal deposits of northwestern Rudny Altai,” Geol. Geofiz. 6, 100–109 (1988).
I. V. Gaskov, E. G. Distanov, N. Yu. Mironova, and V. M. Chekalin, Upper Devonian Sulfide–Base Metal Deposits of Western Rudny Altai, (Nauka, Novosibirsk, 1991) [in Russian].
I. V. Gaskov, V. A. Simonov, V. V. Dranichnikova, and E. O. Terenya, “The composition of solutions and physicochemical conditions of ore precipitation at the sulfide–base metal deposits of northwestern Rudny Altai: fluid inclusion data,” Problems of Geology and Prospecting of Mineral Deposits (Tomsk, 2005), pp. 229–231 [in Russian].
I. V. Gaskov, G. G. Pavlova, A. G. Vladimirov, and I. I. Gvozdev, “Indium and other trace elements in the ores of sulfide–base metal and tin sulfide deposits of Siberia and Far East,” Geology and Mineral-Raw Resources of Siberia3 (1), 67–71 (2014).
I. V. Gaskov, A. G. Vladimirov, A. I. Khanchuk, G. A. Pavlova and V. I. Gvozdev, “Distribution of indium in ores of some base metal and tin–sulfide deposits in Siberia and the Russian Far East,” Geol. Ore Deposits 59 (1), 56–67 (2017).
V. V. Gavrilenko, S. A. Efimenko, G. A. Tkachenko, E. G. Panova, and N. A. Pogrebs, “Geological-structural and mineralogical–geochemical features of the Pravourmiiskoe tin deposit,” Geol. Rudn. Mestorozhd. 6, 34–47 (1992).
P. Gerding and I. Jonsson, “Termochemical studies on metal complexes. VII. Free energy, enthalpy, and entropy changes for stepwise formation of cadmium (II) chloride complexes at different ionic strengths,” Acta Chem. Scand. 22(7), 2247–2254 (1968).
N. A. Gibsher, “Study of ore-forming solutions of the Srednee base metal and Zarechenskoe barite–base metal deposits of Rudny Altai: mineral inclusion data,” Mineralogy of Endogenous Complexes (Novosibirsk, 1975), pp. 67–69 [in Russian].
A. A. Godovikov, Mineralogy (Nedra, Moscow, 1975) [in Russian].
D. V. Grichuk, Thermodynamic Models of Submarine Hydrothermal Systems (Mosk. Gos. Univ., Moscow, 2000) [in Russian].
Y. Hasegawa, T. Shimada, and M. Nitsu, “Solvent extraction of 3B group metal ions from hudrochloric acid with trioctylphosphine oxide,” J. Inorg. Nucl. Chem. 42, 1487–1489 (1980).
C. A. Heinrich, D. Gunther, A. Audetat, T. Ulrich, and R. Frischknecht, “Metal fractionation between magmatic bring and vapor, detected by microanalysis of fluid inclusions,” Geology, 27, 755–758 (1999).
H. C. Helgeson, D. H. Kirkham, and G. G. Flowers, “Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures. IV. Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 5 kb and 600oC,” Am. J. Sci. 281, 1241–1516 (1981).
I. L. Khodakovsky, “Some thermodynamic problems of aqueous solutions at high temperatures and pressures,” Physicochemical Problems of hydrothermal and Magmatic Processes (Nauka, Moscow, 1975), pp. 124–150 [in Russian].
G. R. Kolonin, “Complex compounds of silver, bismuth, and lead with mixed ligands and possibilities of their role in hydrothermal ore formation,” Studies on Experimental Mineralogy (Novosibirsk, 1978), pp. 176–182 [in Russian].
P. G. Korostelev, B. I.Semenyak, and A. M. Kokorin, “Indium in Far East ore deposits. Strategy of use and development of mineral-raw base of trace metals in Russia in 21th century,” Proc. International Symp., Moscow, Russia (VIMS MPR, Moscow, 1998), pp. 77–79 [in Russian].
D. A. Kulik, T. Wagner, S. V. Dmytrieva, G. Kozakowski, F. F. Hingerl, K. V. Chudnenko, and U. Berner, “GEM–Selektor geochemical modeling package: revised algorithm and GEMS3K numerical kernel for coupled simulation codes,” Comp. Geosci. 17, 1–24 (2013).
A. A. Mokeev, E. V. Kozlovsky, and V. P. Vasil’ev, Change of enthalpy and heat capacity in the reactions of formation of simple and mixed chloride–bromide mercury II complexes,” Proc. 8th All-Union Conference on Calorimetry and Chemical Thermodynamics, Ivanova, Russia, 1979), Vol. 1, p.185.
H. Murakami and Sh. Ishihara, “Trace elements of indium–bearing sphalerite from tin–polymetallic deposits in Bolivia, China and Japan: a femto–second LA–ICP–MS study,” Ore Geol. Rev.53, 223–243 (2013).
G. B. Naumov, V. N. Ryzhenko, and I. L. Khodakovsky, Textbook on Thermodynamic Values (Atomizdat, Moscow, 1971) [in Russian].
G. G. Pavlova and A. A. Borovikov, “Silver–antimony deposits of Central Asia: physico–chemical model of formation and sources of mineralization,” Aust. J. Sci. 57, 755–775 (2010).
G. G. Pavlova, Anh Phan Luu, A. G. Vladimirov, A. S. Borisenko, and Th. Seifert, “Geodynamic setting of indium deposits formation,” International Symposium on Large Igneous Provinces of Asian Mantle Plumes and Metallogeny, (Hanoi, 2013), pp. 41–42.
G. G. Pavlova, A. S. Borisenko, A. V. Prokopiev, A. I. Ivanov, A. A. Borovikov, E. A. Vasyukova, and A. V. Travin, “Indium in tin-bearing deposits of Yakutia,” Granites and Earth’s Evolution. Proc. 2nd International Conference (IGM SO RAN, Novosibirsk, 2014), pp. 164–166 [in Russian].
G. G. Pavlova, S. V. Palessky, A. S. Borisenko, A. G. Vladimirov, Th. Seifert, and Anh. Phan Luu, “Indium in cassiterite and ores of tin deposits of Russia,” Ore Geol. Rev. 66, 99–113 (2015a).
G. G. Pavlova, A. S. Borisenko, A. A. Borovikov, A. V. Prokopiev, and A. I. Ivanov, “Indium in tin and Sn–sulfide ores of the Deputatsky ore district (Yakutia),” Absracts of the Joint Assembly AGU–GAC–MAC–CGU, Montreal, Canada, (Montreal, 2015b), Abstract ID: 33272, 297–298 (2015b).
G. G. Pavlova, A. G. Vladimirov, V. I. Gvozdev, P. G. Korostelev, B. I. Semenyak, V. G. Gonevchuk and P. A. Tishin, “In-bearing potential of tin‒sulfide mineralization in ore deposits of the Russian Far East,” Dokl. Earth Sci. 471(1), 1118–1122 (2016).
V. I. Popova, V. A. Popov, P. G. Korostelev, and V. V. Orlovsky, Mineralogy of Ores of the W–Sn Tigrinoe Deposit, Sikhote Alin, and Prospects of its Exploration (Inst. Mineral. UO RAN, Yekaterinburg, 2013) [in Russian].
M. H. Reed, “Calculation of multicomponent chemical equlibria and reaction processes in systems involving minerals, gases and an aqueous phase,” Geochim. Cosmochim. Acta 46, 513–528 (1982).
S. M. Rodionov, Tin Metallogeny of East Russia (Nauka, Moscow, 2005) [in Russian].
Schwarz–Schampera U. and P. M. Herzig, Indium: Geology, Mineralogy, and Economics (Springer–Verlag, Berlin–Heidelberg, 2002).
T. Ryhl, “Thermodynamic properties of indium (III) halogenide and thiocyanate complexes in aqueous solution,” Acta Chem. Scand. 23, 2667–2676 (1969).
Th. Seifert and D. Sandmann, “Mineralogy and geochemistry of indium–bearing polymetallic vein–type deposits: implication for host minerals from the Freiberg district, Eastern Erzgebirge, Germany,” Ore Geol. Rev. 28, 1–31 (2006).
B. I. Semenyak, A. P. Nedashkovsky, and N. N. Nikulin, “Indium minerals in the ores of the Pravourmiiskoe deposit, Russian Far East,” Geol. Rudn. Mestorozhd. 36 (4), 155–163 (1994).
I. P. Shcherban, Yu. A. Dolgov, G. A. Borovikova, and N. A. Gibsher, “Physicochemical conditions of the formation of the Rubtsovskoe sulfide–base metal deposit, Rudny Altai: thermodynamic and thermobarometric data,” Geol. Geofiz. 1, 84–92 (1980).
E. L. Shock, D. C. Sassany, M. Willis, D. A. Sverjensky, “Inorganic species in geologic fluids: correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes,” Geochim. Cosmochim. Acta 61, 907–950 (1997).
V. A. Simonov, N. S. Bortnikov, and A. P. Lisitsin, Physicochemical conditions of mineral formation in the modern Vienna Woods hydrothermal buildup, back-arc Manus Basin, Pacific Ocean,” Metallogeny of Ancient and Modern Oceans-2002. Formation and Exploration of Deposits in Ophiolite Zones(Ins. Mineral. Ural Otd. Ross. Akad. Nauk, Miass, 2002), pp. 61–68 [in Russian].
N. Sunden, “On the complex chemistry of the indium ion: Part IV. An investigation of the chloride and sulfate systems by ion exchangers,” Svensk Kemisk Tidskrift 66, 173–178 (1954).
T. M. Sushchevskaya and B. N. Ryzhenko, “Simulation of mixing of fluids from different sources during cassiterite deposition,” Geochem. Int. 40 (2), 155–163 (2002).
D. A. Sverjensky, E. L. Shock, and H. C. Helgeson, “Prediction of the thermodynamic properties of aqueous metal complexes to 1000°C and 5 kb,” Geochim. Cosmochim. Acta 61(7), 1359–1412 (1997).
J. C. Tanger IV and H. C. Helgeson, “Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: revised equations of state for standard partial molal properties of ions and electrolytes,” Am. J. Sci. 288, 19–98 (1988).
E. O. Terenya, V. A. Simonov, and V. V. Zaikov, Physicochemical conditions of hydrothermal mineral formation at the Kyzyl Tash sulfide deposit, Eastern Tuva,” Metallogeny of Ancient and Modern Oceans –2003.Formation and Exploration of Deposits in the Island-Arc Systems(Inst. Mineral. Ural. Otd. Ross. Akad. Nauk, Miass, 2003), pp. 120–127 [in Russian].
M. K. Tivey, “Generation of seafloor hydrothermal vent fluids and associated mineral deposits,” Oceanography 20 (1), 50–65 (2007).
K. Tunaboylu and G. Schwarzenbach, “Die loslichkeit von indiumsulfid,“ Chimia (Switzerland) 24, 424–427 (1970).
D. R. Turner, M. Whitfield, and A. G. Dickson, “The equilibrium speciation of dissolved components in freshwater and seawater at 25°C and 1 atm pressure,“ Geochim. Cosmochim. Acta 45, 855–881 (1981).
S. Vavra and N. P.Rudenko, “Indium sorption by cationite KU–2 and stability constants of indium chloride complexes in aqueous–ethanol solutions,” Vestn. Mosk. Univ., Ser. II. Khimiya 6, 14–17 (1964).
S. A. Wood and I. M. Samson, “The aqueous geochemistry of gallium, germanium, indium and scandium,“ Ore Geol. Rev. 28, 57–102 (2006).
Funding
This work was supported by the State Task (project no. 0330–2016–0001) and Russian Foundation for Basic Research (project no. 14–05–00191).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by M. Bogina
Rights and permissions
About this article
Cite this article
Gaskov, I.V., Gushchina, L.V. Physicochemical Conditions of the Formation of Elevated Indium Contents in the Ores of Tin–Sulfide and Base-Metal Deposits in Siberia and Far East: Evidence from Thermodynamic Modeling. Geochem. Int. 58, 291–307 (2020). https://doi.org/10.1134/S0016702920030052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702920030052