Abstract—
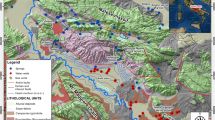
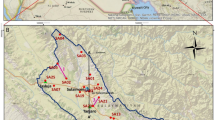
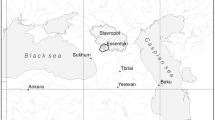
The paper summarizes newly obtained data on the chemical and isotopic composition of groundwaters in the southern part of the Kuznetsk Basin. The territory is demonstrated to host groundwaters of three types, which were distinguished according to the chemical, isotopic, and gas composition of the waters. The waters of type 1 are widespread in areas of sluggish water exchange in coal-bearing terrigenous rocks, bear mineralization of 0.2–4.7 g/L, have pH 7–10, and N2–CH4 and CH4–N2 gas composition. Their source is biogenic (coal) with δ13C from –17.1 to –4.2‰. The sodic waters of type 2 were found relatively recently and only locally, are hosted in the same rocks but at more hampered water exchange, and have mineralization of 5–25 g/L, pH 7.7–10, and CH4 gas composition. These waters are noted for an anomalously isotopically heavy isotopic composition of their carbon δ13С(\({\text{HCO}}_{3}^{ - }\)) = 0.2–30.9‰ and δ13С(CO2) = –3.2–22.3‰. The source of their carbon is also biogenic, but long-lasting interaction in the water–coal system resulted in significant carbon fractionation, with isotopically lighter carbon fractionated into CH4 (δ13С = –67.3 to ‒43.3‰) and isotopically heavier one preferably coming to CO2. The waters of type 3 occur only at certain sites, for example, the Tersinskii CO2 waters. These waters have mineralization of 2–5.5 g/L, pH 6.2–6.7, and CO2 gas composition. Their δ13С(\({\text{HCO}}_{3}^{ - }\)) = –4.3 to –4.1‰ and δ13С(CO2) = –12.3 to –3.9‰ suggest a mixed source of their carbon. This source was mostly deep-seated, not genetically related to waters. All of the sodic waters belong to the infiltration type according to their δ18O and δD, with the waters of type 2 showing a positive oxygen shift. The sodic waters of all three types are shown not to be in equilibrium with many primary aluminosilicates but are oversaturated with respect to carbonates, montmorillonites, illite, chlorite, and sometimes even albite and microcline (waters of type 2). The waters of all of the three types were generated by the same mechanism: by dissolving aluminosilicate minerals in coal-bearing rocks (with which the waters are not in equilibrium) and the simultaneous precipitation of carbonates. The reasons for certain differences between the waters are their interaction with rocks and coal (waters of type 2), which increases their mineralization and makes their carbon and oxygen isotopic compositions heavier, or the addition of carbon dioxide from an external source (waters of type 3), which acidifies the waters.
Similar content being viewed by others
REFERENCES
M. P. Andreeva and E. V. Domrocheva, “Ecological-geochemical state of natural waters of active water exchange zones of the southern Kuznetsk basin,” Izv. Tomsk. Politekhn. Univ. 311(1), 123–133. (2007)
C. A. J. Appelo and D. Postma, Geochemistry, Groundwater and Pollution (A.A. Balkema, Rotterdam, 1994).
R. Blake, “The origin of high sodium bicarbonate waters in the Otway Basin, Victoria, Australia,” Proceed. of 6th Int. Symp. on Water-Rock. Interaction (Brookfield, Rotterdam, 1989), pp. 8–85.
K. M. Christian, L. K. Lautz, G. D. Hoke, Z. Lu, and J. Kessler, “Methane occurrence is associated with sodium-rich valley waters in domestic wells overlying the Marcellus shale in New York State,” Water Resour. Res. 52 (1), 206–226 (2016).
S. Colding, C. J. Boreham, and J. S. Esterle, “Stable isotope geochemistry of coal bed and shale gas and related production waters: a review,” Int. J. Coal Geol. 120, 24–40 (2013).
H. Craig, “Standard for reporting concentrations of deuterium and oxygen-18 in natural waters,” Science 133, 1833–1834 (1961).
E. V. Domrocheva, Extended Abstract of Candidate’s Dissertation in Geology and Mineralogy (TPU, Tomsk, 2005).
E. V. Domrocheva, O. E. Lepokurova, and D. A. Sizikov, “Geochemical characteristics of groundwaters of the Naryk–Ostashkino area, Kuznetsk Basin, Izv. Tomsk. Politekhn. Univ., 325 (1), 94–101 (2014).
G. Faure, Principles of Isotope Geology (Wiley, New York, 1986).
A. A. Feyzullayev and U. A. Movsumova, “The nature of the isotopically heavy carbon of carbon dioxide and bicarbonates in the waters of mud volcanoes in Azerbaijan,” Geochem. Int. 48 (5), 517–522 (2010).
E. M. Galimov, Geochemistry of Stable Carbon Isotopes (Nedra, Moscow, 1968) [in Russian].
R. M. Garrels, and C. L. Christ, Solutions, Minerals, and Equilibria (Harper and Row, New York, 1965).
A. I. Gavrishin, “Genesis of low mineralized soda waters in the Donets Basin, Dokl. Earth Sci. 405 (8), 1183–1185 (2005).
Geology of Coal Deposits of the USSR, Ed. by A. K. Matveeva (MGU, Moscow, 1990) [in Russian].
Hydrogeology of the USSR.Volume 17.Kemerovo District and Altai Krai, Ed. by M. A. Kuznetsova and O. V. Postnikova (Nedra, Moscow, 1972) [in Russian].
J. Jankowski and B. McLean, “Origin of sodium–bicarbonate waters in the south-eastern part of the Great Artesian Basin: influx of magmatic CO2,” in 10th International Symposium on Water-Rock Interaction1, 541–544 (2001).
A. M. Karasevich, V. T.Khryukin, B. M. Zimakov, N. G. Matveenko, S. S. Zolotykh, V. G. Natura, and T. S. Popova, Kuznetsk Basin as the Largest Raw Base of methane extraction from coal seams (Akad. Gornykh Nauk, Moscow, 2001) [in Russian].
V. A. Kazantsev, Problems of Pedohalogenesis (Nauka, Novosibirsk, 1998) [in Russian].
K. Kimura, “Mechanism of the forming of ground water with high content of sodium bicarbonate onto the plains part of the formation Kobe (Japan),”Groundwater Hydrol. 32 (1), 5–16 (1992).
Yu. G. Kopylova, O. E. Lepokurova, and O. G. Tokarenko, “Formation conditions of the chemistry of Tersinskie carbonic-acid mineral water,” Water Res. 36 (5), 577–585 (2009).
Yu. G. Kopylova, O. E. Lepokurova, O. G. Tokarenko, and S. L. Shvartsev, “Chemical composition and genesis of the carbonic-acid mineral waters of the Tersinskoe Deposit (Kuzbass),” Dokl. Earth Sci. 436 (2) 284–289 (2011).
S. R. Krainov, B. N. Ryzhenko, and V. M. Shvets, Geochemistry of Groundwaters. Theoretical, Applied, and Ecological Aspects, 2nd Ed. (Nauka, Moscow, 2012) [in Russian].
V. Yu. Lavrushin, I. S. Guliev, O. E. Kikvadze, Ad. A. Aliev, B. G. Pokrovsky, and B. G. Polyak, “Waters from mud volcanoes of Azerbaijan: isotopic–geochemical properties and generation environments,”Lithol. Miner. Resour.50 (1), 1–25 (2015).
V. Yu. Lavrushin, Underground fluids of the Greater Caucasus and its Framing (GEOS, Moscow, 2012) [in Russian].
O. E. Lepokurova, S. L. Shvartsev, and A. N. Pyryaev, Stable carbon isotopes of water-soluble gases of carbonaceous sediments of the Naryk–Ostashkino area (Kuznetsk Basin), in Proceedings of 2nd All-Russia Conference with International Participance ‘Geological Evolution of Water–Rock Interaction, Vladivostok, Russia,2015 (Dal’nauka, Vladivostok, 2015), pp. 393–396 [in Russian].
G. Matthess, F. H. Frimmel, P. Hursch, H. D. Schulz, and E. Usdowski, Progress in Hydrogeochemistry (Springer-Verlag, Berlin, 1992).
F. May, “Forward modelling of complex water evolution–soda waters in Northland, New Zealand,” 9th International Symposium on Water-Rock Interaction, 885–888 (1998).
V. E. Ol’khovatenko, Engineering Geology of the Coal Deposits of the Kuznetsk Basin (TGASU, Tomsk, 2014) [in Russian].
A. M. Ovchinnikov, G. M. Rogov, and L. A. Solomko, “A new area of the development of carbonic mineral waters of the Kuznetsk Basin,” Izv. Vyssh, Uchebn. Zaved. Geol. Razvedka, No. 11, 71–76 (1964).
E. V. Pinneker, Problems of Regional Geology (Nauka, Moscow, 1977) [in Russian].
V. A. Polyakov, V. T. Dubinchik. E. V. Golubkova, V. A. L’gotin, Yu. V. Makushin, and K. M. Makarova, Isotope studies of groundwaters on the Tomskii test site,” Razved. Okhr. Nedr, No. 11, 47–52 (2008).
O. P. Polyansky, V. V. Reverdatto, and A. N. Fomin, “Model reconstructions of subsidence in the Kuznetsk sedimentary basin,” Russ. Geol. Geophys. 45 (6), 635–644 (2004).
V. G. Popov and R. F. Abdrakhmanov, Ion Exchange Concept in the Genetic Hydrogeochemistry (Gilem, Ufa, 2013) [in Russian].
G. M. Rogov and V. K. Popov, Hydrogeology and Catagenesis of rocks of the Kuznetsk Basin(TGU, Tomsk, 1985) [in Russian].
O. G. Tokarenko, “Groundwaters in the central part of the Kuznetsk Basin: chemical composition within diverse landscape areas,” Izv. Tomsk. Politekhn. Univ. 321 (1), 169–174 (2012).
S. Sharma and C. D. Frost, “An innovative approach for tracing coalbed natural gas produced water using stable isotopes of carbon and hydrogen,” Groundwater 46 (2) 329–334 (2008).
S. L. Shvartsev and Y. Wang, “Geochemistry of sodic waters in the Datong Intermountain Basin, Shanxi Province, Northwestern China,” Geochem. Int. 44 (10), 1015-1026 (2006).
S. L. Shvartsev, “Hydrogeochemical conditions of formation of dawsonite mineralization in the Berezovoyarka area, Kuznetsk Basin,” Geochem. Int. 42 (10), 938–949 (2004).
S. L. Shvartsev, E. V. Domrocheva, and N. M. Rasskazov, “Geochemistry and formation of sodic waters of the Kuznetsk Basin,” Izv. Tomsk. Politekhn. Univ. 318 (1), 128–134 (2011).
S. L. Shvartsev, Hydrogeochemistry of Supergene Zone (Nedra, Moscow, 1998) [in Russian].
S. L. Shvartsev, O. E. Lepokurova, V. A. Ponomarchuk, E. V. Domrocheva and D. A. Sizikov, “Anomalous composition of carbon isotopes in underground alkaline waters of the Kuznetsk basin,” Dokl. Earth Sci. 469 (2),877–881 (2016).
S. L. Shvartsev, V. T. Khryukin, E. V. Domrocheva, K. I. Kuzevanov, N. M. Rasskazov, T. S. Popova, O. E. Lepokurova, and E. V. Shvachko, “Hydrogeology of the Erunakovo region of the Kuznetsk basin in the context of coal methane formation and mining,” Russ. Geol. Geophys. 47 (7), 878–889 (2006).
S. L. Shvartsev, B. N. Ryzhenko, V. A. Alekseev, E. M. Dutova, I. A.Kondrat’eva, and O. E. Lepokurova, Geological Evolution and Self-Organization of the Water–Rock System. Volume 2. “Water–Rock” System in the Supergene Zone (SO RAN, Novosibirsk, 2007) [in Russian].
Z. Zhu, J. Chen, and Y. Zeng, “Abnormal positive δ13C values of carbonate in Lake Caohai, southwest China, and their possible relation to lower temperature,” Quat. Int. 286, 85–93 (2013).
ACKNOWLEDGMENTS
The author thanks S.L. Shvartsev for consultations and E.V. Domrocheva for materials provided for this research. This study was financially supported by Russian Science Foundation (project no. 17-17-01158), the Russian Foundation for Basic Research, project no. 17-05-00042), and the Program of Fundamental Research of the Russian Academy of Science for 2013–2020 (project no. IX.131.3.3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Kurdyukov
Rights and permissions
About this article
Cite this article
Lepokurova, O.E. Sodic Groundwaters in the Southern Kuznetsk Basin: Isotopic and Chemical Characteristics and Genesis. Geochem. Int. 56, 934–949 (2018). https://doi.org/10.1134/S0016702918090069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702918090069