Abstract
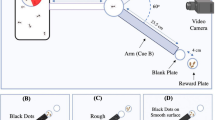
Miniaturization leads to significant changes in the structure of the insect brain. The question of how these changes affect brain functioning is of great interest for understanding the evolution of the brain and cognitive characteristics in animals. Learning ability was previously described for several microinsect species, but comparative analysis of these cases is difficult due to the variable testing conditions. We developed a setup based on the Morris water maze design for aversive training of various miniature insects using visual stimuli. This setup was used to study the behavior of Thrips tabaci (Thysanoptera, Thripidae), and the ability for associative learning and memory formation was demonstrated in thrips for the first time.







Similar content being viewed by others
REFERENCES
Arthur, A.P., Associative learning in Itoplectis conquisitor (Say) (Hymenoptera: Ichneumonidae), Can. Entomol., 1966, vol. 98, no. 2, p. 213.
Avarguès-Weber, A., d’Amaro, D., Metzler, M., Finke, V., Baracchi, D., and Dyer, A.G., Does holistic processing require a large brain? Insights from honeybees and wasps in fine visual recognition tasks, Front. Psychol., 2018, vol. 9, p. 1.
Baeder, J.M. and King, B.H., Associative learning of color by males of the parasitoid wasp Nasonia vitripennis (Hymenoptera: Pteromalidae), J. Insect Behav., 2004, vol. 17, no. 2, p. 201.
Beavers, J.B., Shaw, J.G., and Hampton, R.B., Color and height preference of the citrus thrips in a navel orange grove, J. Econ. Entomol., 1971, vol. 64, no. 5, p. 1112.
Beckham, C.M., Color preference and flight habits of thrips associated with cotton, J. Econ. Entomol., 1969, vol. 62, no. 3, p. 591.
Bowdish, T.I. and Bultman, T.L., Visual cues used by mantids in learning aversion to aposematically colored prey, Am. Midl. Nat., 1993, vol. 129, no. 2, p. 215.
Brandeis, R., Brandys, Y., and Yehuda, S., The use of the Morris water maze in the study of memory and learning, Int. J. Neurosci., 1989, vol. 48, p. 29.
Buehlmann, C., Mangan, M., and Graham, P., Multimodal interactions in insect navigation, Anim. Cognit., 2020, vol. 23, no. 6, p. 1129.
Chilaka, N., Perkins, E., and Tripet, F., Visual and olfactory associative learning in the malaria vector Anopheles gambiae sensu stricto, Malaria J., 2012, vol. 11, no. 27, p. 1.
Chittka, L. and Niven, J., Are bigger brains better? Curr. Biol., 2009, vol. 19, no. 21, p. 995.
Davis, R.L. and Zhong, Y., The biology of forgetting – a perspective, Neuron, 2017, vol. 95, no. 3, p. 490.
De Agrò, M., Oberhauser, F.B., Loconsole, M., Galli, G., Dal Cin, F., Moretto, E., and Regolin, L., Multi-modal cue integration in the black garden ant, Anim. Cognit., 2020, vol. 23, no. 6, p. 1119.
Demirel, N. and Yildirim, A., Attraction of various sticky color traps to Thrips tabaci Lindeman (Thysanoptera: Thripidae) and Empoasca decipiens Paoli (Homoptera: Cicadellidae) in cotton, J. Entomol., 2008, vol. 5, no. 6, p. 389.
Disterhoft, J., Nurnberger, J., and Corning, W.C., “P–R” differences in intact cockroaches as a function of testing interval, Psychon. Sci., 1968, vol. 12, no. 5, p. 205.
Dukas, R. and Duan, J.J., Potential fitness consequences of associative learning in a parasitoid wasp, Behav. Ecol., 2000, vol. 11, no. 5, p. 536.
Eberhard, W.G., Miniaturized orb-weaving spiders: behavioural precision is not limited by small size, Proc. R. Soc. B Biol. Sci., 2007, vol. 247, no. 1622, p. 2203.
Elimem, M. and Chermiti, B., Color preference of Frankliniella occidentalis (Pergande) (Thysanoptera; Thripidae) and Orius sp. (Hemiptera; Anthocoridae) populations on two rose varieties, Floricult. Ornamental Biotechnol., 2012, vol. 7, no. 1, p. 94.
Farahani, H.K., Ashouri, A., Goldansaz, S.H., Shapiro, M.S., Golshani, A., and Abrun, P., Associative learning and memory duration of Trichogramma brassicae, Progr. Biol. Sci., 2014, vol. 4, no. 1, p. 87.
Giurfa, M. and Malun, D., Associative mechanosensory conditioning of the proboscis extension reflex in honey-bees, Learn. Mem., 2004, vol. 11, no. 3, p. 294.
Grimaldi, D. and Engel, M.S., Evolution of the Insects, New York: Cambridge University Press, 2005.
Horridge, G.A., Learning of leg position by the ventral nerve cord in headless insects, Proc. R. Soc. B Biol. Sci., 1962, vol. 157, no. 966, p. 33.
Huigens, M.E., Pashalidou, F.G, Qian, M-H., Bukovinszky, T., Smid, H.M., van Loon, J.J.A., Dicke, M., and Fatouros, N.E., Hitch-hiking parasitic wasp learns to exploit butterfly antiaphrodisiac, Proc. Natl. Acad. Sci. U.S.A., 2009, vol. 106, no. 3, p. 820.
Iakovlev, I. and Reznikova, Z., Red wood ants display natural aversive learning differently depending on their task specialization, Front. Psychol., 2019, vol. 10, no. 710, p. 1.
Kaissling, K.-E., Insect olfaction, in Handbook of Sensory Physiology, Vol. 4: Chemical Senses, No. 1: Olfaction, Berlin etc.: Springer, 1971, p. 351.
Kartsev, V.M. and Mazokhin-Porshnyakov, G.A., Diversity of individually acquired responses in insects, Biol. Nauki, 1989, vol. 3, p. 5.
Keasar, T., Ney-Nifle, M., and Mangel, M., Evidence for learning of visual host-associated cues in the parasitoid wasp Trichogramma thalense, Isr. J. Zool., 2000, vol. 46, no. 3, p. 243.
Loukola, O.J., Perry, C.J., Coscos, L., and Chittka, L., Bumblebees show cognitive flexibility by improving on an observed complex behavior, Science, 2017, vol. 355, no. 6327, p. 833.
Loukola, O.J., Gatto, E., Híjar-Islas, A.C., and Chittka, L., Selective interspecific information use in the nest choice of solitary bees, Anim. Biol., 2020, vol. 70, no. 2, p. 215.
Lu, F.M., Color preference and using silver mulches to control the onion thrips, Thrips tabaci Lindeman, Chin. J. Entomol., 1990, vol. 10, no. 3, p. 337.
MaBouDi, H.D., Solvi, C., and Chittka, L., Bumblebees learn a relational rule but switch to a win-stay/lose-switch heuristic after extensive training, Front. Behav. Neurosci., 2020, vol. 14, p. 1.
Makarova, A.A. and Polilov, A.A., Peculiarities of the brain organization and fine structure in small insects related to miniaturization. 4. Thrips (Thysanoptera, Thripidae), Entomol. Rev., 2017, vol. 97, no. 3, p. 302.
Makarova, A.A. and Polilov, A.A., Structure and ultrastructure of the Acrotrichis grandicollis (Coleoptera: Ptiliidae) compound eyes and the eye features related to miniaturization, Dokl. Biol. Sci., 2018, vol. 480, p. 97.
Makarova, A.A., Polilov, A.A., and Chklovskii, D.B., Small brains for big science, Curr. Opin. Neurobiol., 2021, vol. 71, p. 77.
Mansur, B.E., Rodrigues, J.R.V., and Mota, T., Bimodal patterning discrimination in harnessed honey bees, Front. Psychol., 2018, vol. 9, p. 1.
Margulies, C., Tully, T., and Dubnau, J., Deconstructing memory in Drosophila, Curr. Biol., 2005, vol. 15, no. 17, p. 700.
Matsumoto, Y. and Mizunami, M., Lifetime olfactory memory in the cricket Gryllus bimaculatus, J. Comp. Physiol. A, 2002, vol. 188, no. 4, p. 295.
Mazokhin-Porshnyakov, G.A., Learning ability and visual cue integration in insects, Entomol. Obozr., 1968, vol. 47, no. 36, p. 362.
Mazokhin-Porshnyakov, G.A., Visual cue integration as an example of solving abstract problems in bees, Zool. Zh., 1969, vol. 48, p. 1125.
Mazokhin-Porshnyakov, G.A., Visual orientation and navigation in insects, in Prostranstvennaya orientatsiya zhivotnykh (Spatial Orientation of Animals), 1970a, p. 24.
Mazokhin-Porshnyakov, G.A., Is insect behavior controlled by instinct alone? Priroda, 1970b, no. 5, p. 55.
Mazokhin-Porshnyakov, G.A. and Kazyakina, V.I., Morphological description of compound eyes and ocelli in thrips (Thysanoptera), Biol. Nauki, 1983, no. 1, p. 57.
McGuire, S.E., Deshazer, M., and Davis, R.L., Thirty years of olfactory learning and memory research in Drosophila melanogaster, Progr. Neurobiol., 2005, vol. 76, no. 5, p. 328.
Minoli, S., Cano, A., Pontes, G., Magallanes, A., Roldán, N., and Barrozo, R.B., Learning spatial aversion is sensoryspecific in the hematophagous insect Rhodnius prolixus, Front. Psychol., 2018, vol. 9, p. 1.
Morris, R.G.M., Spatial localization does not require the presence of local cues, Learn. Motiv., 1981, vol. 12, no. 2, p. 239.
Nityananda, V. and Chittka, L., Different effects of reward value and saliency during bumblebee visual search for multiple rewarding targets, Anim. Cogn., 2021, vol. 24, no. 4, p. 803.
Ofstad, T.A., Zuker, C.S., and Reiser, M.B., Visual place learning in Drosophila melanogaster, Nature, 2011, vol. 474, no. 7350, p. 204.
Polilov, A.A., Small is beautiful: Features of the smallest insects and limits to miniaturization, Annu. Rev. Entomol., 2015, vol. 60, no. 1, p. 103.
Polilov, A.A., Makarova, A.A., and Kolesnikova, U.K., Cognitive abilities with a tiny brain: neuronal structures and associative learning in the minute Nephanes titan (Coleoptera: Ptiliidae), Arthropod Str. Dev., 2019, vol. 48, p. 98.
Pomaville, M.B. and Lent, D.D., Multiple representations of space by the cockroach, Periplaneta americana, Front. Psychol., 2018, vol. 9, p. 1.
Prokopy, R.J., Averill, A.L., Cooley, S.S., and Roitberg, C.A., Associative learning in egglaying site selection by apple maggot flies, Science, 1982, vol. 218, no. 4567, p. 76.
Rasnitsyn, A.P. and Quicke, D.L.J. (Eds.), History of Insects, Dordrecht: Kluwer Academic Publishers, 2002.
Reznikova, Z., Spatial cognition in the context of foraging styles and information transfer in ants, Anim. Cogn., 2020, vol. 23, no. 6, p. 1143.
Roper, M., Fernando, C., and Chittka, L., Insect bio-inspired neural network provides new evidence on how simple feature detectors can enable complex visual generalization and stimulus location invariance in the miniature brain of honeybees, PLoS Comput. Biol., 2017, vol. 13, no. 2, p. 1.
Salas, J., Biology and life habits of the onion thrips (Thrips tabaci Linderman), Acta Hortic., 1994, vol. 358, p. 383.
Schleyer, M., Fendt, M., Schuller, S., and Gerber, B., Associative learning of stimuli paired and unpaired with reinforcement: evaluating evidence from maggots, flies, bees, and rats, Front. Psychol., 2018, vol. 9, p. 1.
Schwärzel, M. and Müller, U., Dynamic memory networks: dissecting molecular mechanisms underlying associative memory in the temporal domain, Cell. Mol. Life Sci., 2006, vol. 63, no. 9, p. 989.
Shettleworth, S.J., Varieties of learning and memory in animals, Anim. Behav. Proc., 1993, vol. 19, no. 1, p. 5.
Shull, F., Biology of the thysanoptera. I. Factors governing local distribution, Am. Natur., 1902, vol. 142, p. 161.
Smid, H.M., Wang, G., Bukovinszky, T., Steidle, J.L.M., Bleeker, M.A.K., van Loon, J.J.A., and Vet, L.E.M., Speciesspecific acquisition and consolidation of long-term memory in parasitic wasps, Proc. R. Soc. B Biol. Sci., 2007, vol. 274, no. 1617, p. 1539.
Tully, T. and Quinn, W.G., Classical conditioning and retention in normal and mutant Drosophila melanogaster, J. Comp. Physiol. A, 1985, vol. 157, p. 263.
Vinauger, C., Lallement, H., and Lazzari, C.R., Learning and memory in Rhodnius prolixus: Habituation and aversive operant conditioning of the proboscis extension response, J. Exp. Biol., 2013, vol. 216, no. 5, p. 892.
Vorhees, C.V. and Williams, M.T., Morris water maze: procedures for assessing spatial and related forms of learning and memory, Nat. Protoc., 2006, vol. 1, no. 2, p. 848.
Van der Woude, E., Huigens, M.E., and Smid, H.M., Differential effects of brain size on memory performance in parasitic wasps, Anim. Behav., 2018, vol. 141, p. 57.
Watanabe, H., Kobayashi, Y., Sakura, M., Matsumoto, Y., and Mizunami, M., Classical olfactory conditioning in the cockroach Periplaneta americana, Zool. Sci., 2003, vol. 20, no. 12, p. 1447.
Wessnitzer, J., Mangan, M., and Webb, B., Place memory in crickets, Proc. R. Soc. B Biol. Sci., 2008, vol. 275, p. 915.
Yong, T.-H., Pitcher, S., Gardner, J., and Hoffmann, M.P., Odor specificity testing in the assessment of efficacy and non-target risk for Trichogramma ostriniae (Hymenoptera: Trichogrammatidae), Biocontrol Sci. Technol., 2007, vol. 17, no. 2, p. 135.
ACKNOWLEDGMENTS
We are grateful to Lomonosov Moscow State University students Z. Adaeva, A. Dolgov, N. Motorin, A. Rosinskaya, and A. Melnikova for help in conducting the experiments.
Funding
This work was supported by the Russian Science Foundation (project 19-74-10019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All the applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All the procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Fedorova, M.A., Farisenkov, S.E., Timokhov, A.V. et al. Associative Learning and Memory in Thrips tabaci (Thysanoptera, Thripidae). Entmol. Rev. 102, 769–781 (2022). https://doi.org/10.1134/S0013873822060021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0013873822060021