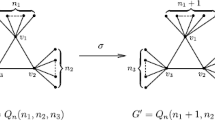Abstract
One of the techniques for theoretically determining enthalpies of formation of organic compounds is the method of homodesmic reactions. In this work, a graph theory interpretation of acyclic chemical compounds was presented, and an algorithm for constructing a basis of homodesmic reactions was developed, which makes it possible to use a homodesmic approach to calculating the enthalpy of formation. Using the developed algorithm was exemplified by building the basis of homodesmic reactions for the butyramide molecule.
Similar content being viewed by others
References
Stull, D.R., Westrum, E.F., and Sinke, G.C., The Chemical Thermodynamics of Organic Compounds, Wiley & Sons, 1969. Translated under the title Khimicheskaya termodinamika organicheskikh soedinenii, Moscow Mir, 1971.
Pedley, J.B., Naylor, R.D., and Kirby, S.P., Thermochemical Data of Organic Compounds, London Chapman & Hall, 1986.
Khursan, S.L., Bashkirsk. Khim. Zh., 1997, vol. 4, no. 1, pp. 37–41.
Hehre, W.J., Radom, L., Schleyer, P.v.R., and Pople, J.A., Ab Initio Molecular Orbital Theory, New York John Wiley & Sons, 1986.
Khursan, S.L., Vestn. Bashkirsk. Univ., 2014, vol. 19, no. 2, pp. 395–401.
Khursan, S.L., Ismagilova, A.S., Akhmerov, A.A., and Spivak, S.I., Zh. Fiz. Khim., 2016, vol. 90, no. 4, pp. 569–575.
Benson S.W., Thermochemical Kinetics, Wiley and Sons, 1968. Translated under the title Termokhimicheskaya kinetika, Moscow Mir, 1971.
Spivak, S.I. and Ismagilova, A.S., Dokl. Phys. Chem., 2014, vol. 455, part 2, pp. 53–55.
Ore, O., Graphs and Their Uses, The Mathematical Association of America, 1991. Translated under the title Grafy i ikh primenenie, Moscow KomKniga, 2006.
Afeefy, H.Y., Liebman, J.F., and Stein, S.E., NIST Chemistry Webbook, NIST Standard Reference Database Number 69, Linstrom, P.J. and Mallard, W.G., Eds., Natl. Inst. Stand. Technol., Gaithersburg (Md), 2089. http://webbook.nist.gov.
Curtiss, L.A., Raghavachari, K., Redfern, P.C., et al., J. Chem. Phys. 1998, vol. 109, no. 18, pp. 7764–7776.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., and Fox, D.J., Gaussian 09, Revision C.1, Gaussian, Inc., Wallingford CT, 2009.
Akhmerov, A.A., Ismagilova, A.S., Spivak, S.I., and Khursan, S.L., RF Patent 2015617060, 29.06.2015.
Akhmerov, A.A., Ismagilova, A.S., Spivak, S.I., and Khursan, S.L., RF Patent 2015621003.01.07.2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.L. Khursan, A.S. Ismagilova, S.I. Spivak, 2017, published in Doklady Akademii Nauk, 2017, Vol. 474, No. 4, pp. 454–457.
Rights and permissions
About this article
Cite this article
Khursan, S.L., Ismagilova, A.S. & Spivak, S.I. A graph theory method for determining the basis of homodesmic reactions for acyclic chemical compounds. Dokl Phys Chem 474, 99–102 (2017). https://doi.org/10.1134/S0012501617060033
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012501617060033