Abstract
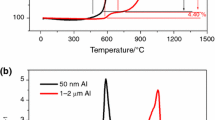
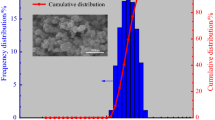
The use of nanosized metal powders is a promising direction in the development of modern energy compositions due to their high reactivity and intense heat release upon contact with an oxidizer and during combustion. The results of a combined analysis (thermogravimetric analysis and differential scanning calorimetry) of Alex aluminum nanopowders and a Al–Cu compound, obtained via electrical explosion of conductors, are presented at constant heating rates of 2, 4, and 20°C /min in air in a temperature range of 30–1300°C . It is revealed that Alex and Al–Cu nanopowders are intensely oxidized when heated in air to a temperature of 600°C due to the oxidizer diffusion through the porous oxide layer Al2O3 and the possible formation of open surfaces of an active metal during a phase change in the crystal lattice of the metal oxide. The Friedman and Kissinger–Akahira–Sunose methods were used to obtain dependences between the activation oxidation energy on the degree of conversion (oxidation) of nanosized metal powders. It is shown that the activation energy of Alex and Al–Cu nanopowders depends on the degree of conversion (oxidation stages) and lies in ranges of 78–307 and 99–430 kJ/mol, respectively.





Similar content being viewed by others
REFERENCES
M. A. Gurevich, K. I. Lapkina, and E. S. Ozerov, “Ignition Limits of Aluminum Particles," Fiz. Goreniya Vzryva 6 (2), 182–176 (1970) [Combust., Expl., Shock Waves 6 (2), 154–157 (1970)].
L. Meda, G. Marra, L. Galfetti, et al., “Nano-Aluminum as Energetic Material for Rocket Propellants," Mater. Sci. Eng. C 27 (5–8), 1393–1396 (2007); DOI: 10.1016/j.msec.2006.09.030.
D. Sundaram, “Metal–Water Mixtures for Propulsion and Energy-Conversion Applications: Recent Progress and Future Directions," Euras. Chem.-Technol. J. 20 (1), 53–62 (2018); DOI: 10.18321/ectj708.
D. A. Rodriguez, E. L. Dreizin, and E. Shafirovich, “Hydrogen Generation from Ammonia Borane and Water Through Combustion Reactions with Mechanically Alloyed Al–Mg Powder," Combust. Flame 162 (4), 1498–1506 (2015); 10.1016/j.combustflame.2014.11.019.
H. Belal, C. W. Han, I. E. Gunduz, et al., “Ignition and Combustion Behavior of Mechanically Activated Al–Mg Particles in Composite Solid Propellants," Combust. Flame 194, 410–418 (2018); DOI: 10.1016/j.combustflame.2018.04.010.
H. Lee, J. H. Kim, S. Kang, et al., “Ignition of Nickel Coated Aluminum Agglomerates Using Shock Tube," Combust. Flame 221, 160–169 (2020); DOI: 10.1016/j.combustflame.2020.07.021.
S. Kim, J. Lim, S. Lee, et al., “Study on the Ignition Mechanism of Ni-Coated Aluminum Particles in Air," Combust. Flame 198, 24–39 (2018); DOI: 10.1016/j.combustflame.2018.07.010.
A. Abraham, H. Nie, M. Schoenitz, et al., “Bimetal Al–Ni Nano-Powders for Energetic Formulations," Combust. Flame 173, 179–186 (2016); DOI: 10.1016/j.combustflame.2016.08.015.
T. Bazyn, N. Glumac, H. Krier, “Reflected Shock Ignition and Combustion of Aluminum and Nanocomposite Thermite Powders," Combust. Sci. Technol. 179 (3), 457–476 (2007); DOI: 10.1080/00102200600637261.
F. Tepper, “Nanosize Powders Produced by Electro-Explosion of Wire and Their Potential Applications," Powder Metal. 43 (4), 320–322 (2000).
M. I. Lerner, A. V. Pervikov, E. A. Glazkova, et al., “Structures of Binary Metallic Nanoparticles Produced by Electrical Explosion of Two Wires from Immiscible Elements," Powder Technol. 288, 371–378 (2016); DOI: 10.1016/j.powtec.2015.11.037.
K. Gnanaprakash, S. R. Chakravarthy, and R. Sarathi, “Combustion Mechanism of Composite Solid Propellant Sandwiches Containing Nano-Aluminium," Combust. Flame 182, 64–75 (2017); DOI: 10.1016/j.combustflame.2017.04.024.
A. G. Korotkikh, I. V. Sorokin, E. A. Selikhova, and V. A. Arkhipov, “Effect of B, Fe, Ti, Cu Nanopowders on the Laser Ignition of Al-Based High-Energy Materials," Combust. Flame 222, 103–110 (2020); DOI: 10.1016/j.combustflame.2020.08.045.
L. De Luca, L. Galfetti, G. Colombo, et al., “Microstructure Effects in Aluminized Solid Rocket Propellants," J. Propul. Power 26 (4), 724–732 (2010); DOI: 10.2514/1.45262.
A. Gromov, L. T. De Luca, A. P. Il’in, et al., “Nanometals in Energetic Systems: Achievements and Future," Int. J. Energ. Mater. Chem. Propul. 13 (5), 399–419 (2014); DOI: 10.1615/IntJEnergeticMaterialsChemProp.2014011255.
Y. Huang, G. A. Risha, V. Yang, and R. A. Yetter, “Effect of Particle Size on Combustion of Aluminum Particle Dust in Air," Combust. Flame 156 (1), 5–13 (2009); DOI: 10.1016/j.combustflame.2008.07.018.
D. Sundaram, V. Yang, and R. A. Yetter, “Metal-Based Nanoenergetic Materials: Synthesis, Properties, and Applications," Prog. Energy Combust. Sci. 61, 293–365 (2017); DOI: 10.1016/j.pecs.2017.02.002.
T. R. Sippel, S. F. Son, and L. J. Groven, “Aluminum Agglomeration Reduction in a Composite Propellant Using Tailored Al/PTFE Particles," Combust. Flame 161 (1), 311–321 (2014); DOI: 10.1016/j.combustflame.2013.08.009.
D. S. Sundaram, P. Puri, and V. Yang, “Pyrophoricity of Nascent and Passivated Aluminum Particles at Nano-Scales," Combust. Flame 160 (9), 1870–1875 (2013); DOI: 10.1016/j.combustflame.2013.03.031.
V. E. Zarko and A. A. Gromov, Energetic Nanomaterials: Synthesis, Characterization, and Application (Elsevier Inc., 2016).
A. V. Pervikov, S. O. Kazantsev, A. S. Lozhkomoev, and M. I. Lerner, “Bimetallic Al–Ag, Al–Cu and Al–Zn Nanoparticles with Controllable Phase Compositions Prepared by the Electrical Explosion of Two Wires," Powder Technol. 372, 136–147 (2020); DOI: 10.1016/j.powtec.2020.05.088.
F. Noor, A. Vorozhtsov, M. Lerner, et al., “Thermal-Chemical Characteristics of Al–Cu Alloy Nanoparticles," J. Phys. Chem. C. 119 (25), 14001–14009 (2015); DOI: 10.1021/acs.jpcc.5b01515.
T. Fournaise, J. Burgain, C. Perroud, et al., “Impact of Formulation on Reconstitution and Flowability of Spray-Dried Milk Powders," Powder Technol. 372, 107–116 (2020); DOI: 10.1016/j.powtec.2020.05.085.
C. Salameh, J. Scher, J. Petit, et al., “Physico-Chemical and Rheological Properties of Lebanese Kishk Powder, a Dried Fermented Milk–Cereal Mixture," Powder Technol. 292, 307–313 (2016); DOI: 10.1016/j.powtec.2016.01.040.
V. A. Arkhipov, S. S. Bondarchuk, A. G. Korotkikh, and M. I. Lerner, “Aluminum Nanopowders: Production Technology and Disperse Characteristics," Tsvet. Met., No. 4, 58–66 (2006).
M. A. Trunov, M. Schoenitz, X. Zhu, and E. L. Dreizin, “Effect of Polymorphic Phase Transformations in Al2O3 Film on Oxidation Kinetics of Aluminum Powders," Combust. Flame 140 (4), 310–318 (2005); DOI: 10.1016/j.combustflame.2004.10.010.
M. A. Trunov, M. Schoenitz, and E. L. Dreizin, “Ignition of Aluminum Powders Under Different Experimental Conditions," Propell., Explos., Pyrotech. 30 (1), 36–43 (2005); DOI: 10.1002/prep.200400083.
S. Vyazovkin, A. K. Burnham, J. M. Criado, et al., “ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data," Thermochim. Acta. 520 (1/2), 1–19 (2011); DOI: 10.1016/j.tca.2011.03.034.
D. Hastings, M. Schoenitz, and E. L. Dreizin, “Highly Reactive Spheroidal Milled Aluminum," Materialia 15, 100959 (2021); DOI: 10.1016/j.mtla.2020.100959.
A. B. Vorozhtsov, M. Lerner, N. Rodkevich, et al., “Oxidation of Nano-Sized Aluminum Powders," Thermochim. Acta. 636, 48–56 (2016); DOI: 10.1016/j.tca.2016.05.003.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Fizika Goreniya i Vzryva, 2022, Vol. 58, No. 2, pp. 38-48.https://doi.org/10.15372/FGV20220204.
Rights and permissions
About this article
Cite this article
Korotkikh, A.G., Godunov, A.B. & Sorokin, I.V. Al–Cu Powder Oxidation Kinetics during Heating in Air. Combust Explos Shock Waves 58, 159–168 (2022). https://doi.org/10.1134/S0010508222020046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0010508222020046