Abstract
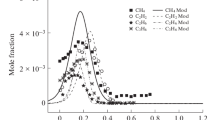
Methyl methacrylate (MMA) is the main pyrolysis product of the widely used polymer polymethyl methacrylate; therefore, a compact mechanism of MMA oxidation is of interest for CFD modeling of flame propagation over this polymer. A reduced mechanism of MMA combustion consisting of 263 elementary reactions involving 66 species was developed based on a detailed chemical-kinetic mechanism of MMA oxidation in flames using Chemical Workbench software. The developed mechanism was validated against experimental data on the speed of premixed MMA/air flame at an equivalence ratio \(0.9 < \phi < 1.3\) and against literature data on the structure of a MMA/O2/Ar (\(\phi\) = 1) flame stabilized on a flat burner at a pressure of 1 atm. The proposed reduced kinetic model for MMA describes the experimental data with satisfactory accuracy, and the modeling results for the full and reduced mechanisms of MMA oxidation are in good agreement with each other in terms of the concentrations of the major flame species and the concentrations of most combustion intermediates, including hydrogen, methane, ethylene, acetylene, propane, acetaldehyde, methyl acrylate, etc.





Similar content being viewed by others
REFERENCES
J. Rabinovitch, E. T. Jens, A. C. Karp, B. Nakazono, A. Conte, and D. A. Vaughan, “Characterization of Polymethylmethacrylate As a Fuel for Hybrid Rocket Motors," Joint Propul. Conf (2018); DOI: 10.2514/6.2018–4530.
O. P. Korobeinichev, L. L. Paletsky, M. B. Gonchikzhapov, R. K. Glaznev, I. E. Gerasimov, Y. K. Naganovsky, I. K. Shundrina, A. Yu. Snegirev, and R. Vinu, “Kinetics of Thermal Decomposition of PMMA at Different Heating Rates and in a Wide Temperature Range," Thermochim. Acta 671, 17–25 (2019).
W. R. Zeng, S. F. Li, and W. K. Chow, “Preliminary Studies on Burning Behavior of Poly(methylmethacrylate) (PMMA)," J. Fire Sci. 20, 297–317 (2002).
K. Seshadri and, F. Williams, “Structure and Extinction of Counterflow Diffusion Flames above Condensed Fuels Comparison between Poly(Methyl Methacrylate) and Its Liquid Monomer, Both Burning in Nitrogen–Air Mixtures," J. Polymer Sci., A: Polymer Chem.167, 1755–1778 (1978).
H. Arisawa and T. B. Brill, “Kinetics and Mechanisms of Flash Pyrolysis of Poly(methylmethacrylate) PMMA," Combust. Flame109, 415–426 (1997).
T. Wang, S. Li, Z. Lin, D. Han, and X. Han, “Experimental Study of Laminar Lean Premixed Methylmethacrylate/Oxygen/Argon Flame at Low Pressure," J. Phys. Chem. 1126, 1219–1227 (2008).
Z. Lin, T. Wang, D. Han, X. Han, S, Li, Y. Li, and Z. Tian, “Study of Combustion Intermediates in Fuel-Rich Methyl Methacrylate Flame with Tunable Synchrotron Vacuum Ultraviolet Photoionization Mass Spectrometry," Rapid Commun. Mass Spectrom. 231, 85–92 (2009).
B. Yang, C. K. Westbrook, T. A. Cool, N. Hansen, and K. Kohse-Höinghaus, “Photoionization Mass Spectrometry and Modeling Study of Premixed Flames of Three Unsaturated C5H8O2 Esters," Proc. Combust. Inst. 34, 443–451 (2013).
S. Dakshnamurthy, D. A. Knyazkov, A. M. Dmitriev, O. P. Korobeinichev, E. J. K. Nilsson, A. A. Konnov, and K. Narayanaswam, “Experimental Study and a Short Kinetic Model for High-Temperature Oxidation of Methyl Methacrylate," Combust. Sci. Technol.191 (10), 789–814 (2019).
M. O. Conaire, H. J. Curran, J. M. Simmie, W. J. Pitz, and C. K. Westbrook, “A Comprehensive Modeling Study of Hydrogen Oxidation," Int. J. Chem. Kinet. 36, 603–622 (2004).
B. Yang, C. K. Westbrook, T. A. Cool, N. Hansen, and K. Kohse-Hoinghaus, “Fuel-Specific Influences on the Composition of Reaction Intermediates in Premixed Flames of three C5H10O2 Ester Isomers," Phys. Chem. Chem. Phys. 13, 6901–6913 (2011).
B. Yang, C. K. Westbrook, T. A. Cool, N. Hansen, and K. Kohse-Hoinghaus, “The Effect of Carbon–Carbon Double Bonds on the Combustion Chemistry of Small Fatty Acid Esters," Z. Phys. Chem. 225, 1293–1314 (2011).
C. K. Westbrook, C. V. Naik, O. Herbinet, W. J. Pitz, and M. Mehl, “Detailed Chemical Kinetic Reaction Mechanism for Biodiesel Components Methyl Stearate and Methyl Oleate," Proc. Combust. Inst.33, 383–389 (2011).
K. Narayanaswamy, H. Pitsch, and P. Pepiot, “A Component Library Framework for Deriving Kinetic Mechanisms for Multi-Component Fuel Surrogates: Application for Jet Fuel Surrogates," Combust. Flame165, 288–309 (2011).
S. A. Rashkovskiy, S. E. Yakush, and A. A. Baranov, “Combustion Stability in a Solid-Fuel Ramjet Engine," J. Phys.: Conf. Ser.1009 (2018); 11th Int. Conf. Aerophysics and Physical Mechanics of Classical and Quantum Systems (APhM-2017), Moscow, November 21–24, 2017; DOI: 10.1088/1742–6596/1009/1/012032.
S. E. Yakush, S. A. Rashkovskiy, and A. I. Bryzgalov, “Combustion in a Solid Fuel Scramjet with Channel Geometry Variation due to Burnout," J. Phys: Conf. Ser. 1250 (2019);12th Int. Conf. Aerophysics and Physical Mechanics of Classical and Quantum Systems (APhM-2018), Moscow, November 27–29, 2018.
S. Dubtsov, T. Ovchinnikova, S. Valiulin, X. Chen, H. E. Manninen, P. P. Aalto, and T. Petäjä, “Laboratory Verification of Aerosol Diffusion Spectrometer and the Application to Ambient Measurements of New Particle Formation," J. Aerosol Sci. 105 (1), 10–23 (2017).
A. A. Onischuk et al., “Determination of the Aerosol Particle Size Distribution by Means of the Diffusion Battery: Analytical Inversion," Aerosol Sci. Technol. 52 (8), 841–853 (2018).
O. P. Korobeinichev, A. G. Shmakov, A. A. Chernov, D. M. Markovich, M. Dulin, and D. K. Sharaborin, “Spatial and Temporal Resolution of the Particle Image Velocimetry Technique in Flame Speed Measurements," Fiz. Goreniya Vzryva 50 (5), 13–21 (2014) [Combust., Expl., Shock Waves 50 (5), 510–517 (2014); https://doi.org/10.1134/S0010508214050025].
T. A. Bolshova, O. P. Korobeinichev, K. V. Toropetskii, A. G. Shmakov, and A. A. Chernov, “Catalytic Effect of Submicron TiO2 Particles on the Methane–Air Flame Speed," Fiz. Goreniya Vzryva 52 (2), 35–48 (2016) [Combust., Expl., Shock Waves 52 (2), 155–166 (2016); https://doi.org/10.1134/S0010508216020040].
“Mechanism Workbench Software," in http://www. kintechlab.com/products/mechanism-workbench/.
I. E. Gerasimov, T. A. Bolshova, I. A. Zaev, A. V. Lebedev, B. V. Potapkin, A. G. Shmakov, and O. P. Korobeinichev, “Reduced Chemical Kinetic Mechanism for Methyl Pentanoate Combustion," Energy Fuels 31 (12), 14129–14137 (2017).
A. M. Dmitriev, K. N. Osipova, D. A. Knyazkov, I. E. Gerasimov, A. G. Shmakov, and O. P. Korobeinichev, “Comparative Analysis of the Chemical Structure of Ethyl Butanoate and Methyl Pentanoate Flames," Fiz. Goreniya Vzryva 54 (2), 3–4 (2018) [Combust., Expl., Shock Waves 54 (2), 125–135 (2018); https://doi.org/10.1134/S0010508218020016].
I. E. Gerasimov, D. A. Knyazkov, A. M. Dmitriev, L. V. Kuibida, A. G. Shmakov, and O. P. Korobeinichev, “Experimental and Numerical Study of the Structure of a Premixed Methyldeconoate/Oxygen/Argon Flame," Fiz. Goreniya Vzryva 51 (3), 3–11 (2015) [Combust., Expl., Shock Waves 51 (3), 51, 285–292 (2015); https://doi.org/10.1134/S0010508215030016].
O. P. Korobeinichev, I. E. Gerasimov, D. A. Knyazkov, A. G. Shmakov, T. A. Bolshova, N. Hansen, C. K. Westbrook, G. Dayma, and B. Yang, “An Experimental and Kinetic Modeling Study of Premixed Laminar Flames of Methyl Pentanoate and Methyl Hexanoate," Z. Phys. Chem. 229 (5), 759–780 (2015).
R. J. Kee, F. M. Rupley, and J. A. Miller, “CHEMKIN-II: A Fortran Chemical Kinetics Package for the Analysis of Gas Phase Chemical Kinetics," Report No. SAND 89–8009B (Sandia National Laboratories, 1989).
R. S. Khare et al., “A Comprehensively Validated Compact Mechanism for Dimethyl Ether Oxidation: An Experimental and Computational Study," Combust. Flame 196, 116–128 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizika Goreniya i Vzryva, 2021, Vol. 57, No. 2, pp. 34–47.https://doi.org/10.15372/FGV20210204.
Rights and permissions
About this article
Cite this article
Bolshova, T.A., Chernov, A.A. & Shmakov, A.G. Reduced Chemical Kinetic Mechanism for the Oxidation of Methyl Methacrylate in Flames at Atmospheric Pressure. Combust Explos Shock Waves 57, 159–170 (2021). https://doi.org/10.1134/S0010508221020040
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0010508221020040