Abstract
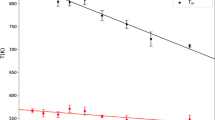
Molecular dynamics modeling of melting of aluminum nanoparticles with the use of the DL POLY simulation package and two types of parametrization of the embedded atom potential is performed. Predicted melting temperatures are compared with available experimental and numerical data. A significant scatter of data (melting temperatures as functions of the nanoparticle size) is noted. The previously proposed semi-empirical model of molecular dynamics for the description of the thermal history of the aluminum nanoparticle is justified. The specific heats obtained in this study ensure a qualitatively correct description of their dependence on temperature and on the crystal rib size.
Similar content being viewed by others
References
A. V. Fedorov and A. V. Shulgin, “Complex Modeling of Melting of an Aluminum Nanoparticle,” Fiz. Goreniya Vzryva 49 (4), 68–75 (2013) [Combust., Expl., Shock Waves 49 (4), 442–449 (2013)].
F. Ercolessi and J. B. Adams, “Interatomic Potentials from First-Principles Calculations: the Force-Matching Method,” Europhys. Lett. 26 (8), 583–588 (1994).
M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids (Oxford Univ. Press, 1991).
D. J. Evans and G. P. Morriss, “Non-Newtonian Molecular Dynamics,” Comput. Phys. Rep. 1 (5), 297–343 (1984).
M. W. Finnis and J. E. Sinclair, “A Simple Empirical N-Body Potential for Transition Metals,” Philos. Mag. A 50 (1), 45–66 (1984).
W. Smith and T. R. Forester, “DL POLY 2.0: A General-Purpose Parallel Molecular Dynamics Simulation Package,” J. Mol. Graphics 14, 136–141 (1996).
F. Ercolessi, “A Molecular Dynamics Primer,” Int. School for Advanced Studies (SISSA-ISAS), Trieste, Italy, 1997.
D. C. Rapaport, The Art of Molecular Dynamics Simulation (Cambridge Univ. Press, 2004).
S. Alavi and D. L. Thompson, “Simulations ofMelting of Polyatomic Solids and Nanoparticles,” Mol. Simulation 32 (12–13), 999–1015 (2006).
P. Puri and V. Yang, “Effect of Particle Size on Melting of Aluminum at Nano Scales,” J. Phys. Chem. C 111, 11776–11783 (2007).
J. Eckert, J. C. Holzer, C. C. Ahn, Z. Fu, and W. L. Johnson, “Melting Behavior of Nanocrystalline Aluminum Powders,” Nanostruct. Mater. 2 (4), 407–413 (1993).
S. Alavi and D. L. Thompson, “Molecular Dynamics Simulations of the Melting of Aluminum Nanoparticles,” J. Phys. Chem. A 110, 1518–1523 (2006).
https://sites.google.com/site/eampotentials/Home.
S. L. Lai, J. R. A. Carlsson, and L. H. Allen, “Melting Point Depression of Al Clusters Generated during the Early Stages of Film Growth: Nanocalorimetry Measurements,” Appl. Phys. Lett. 72 (9), 1098–1100 (1998).
G. Chauhan, “Influence of Alumina Shell on Nano Aluminum Melting Temperature Depression,” in A Thesis in Mechanical Engineering (Texas Tech. Univ., 2007).
Y. F. Zhu, J. S. Lian, and Q. Jiang, “Modeling of the Melting Point, Debye Temperature, Thermal Expansion Coefficient, and the Specific Heat of Nanostructured Materials,” J. Phys. Chem. C 113, 16896–16900 (2009).
H. C. Andersen, “Molecular Dynamics Simulations at Constant Pressure and/or Temperature,” J. Chem. Phys. 72 (4), 2384–2393 (1980).
A. P. Babichev, N. A. Babushkina, A. M. Bratkovskii, et al. Physical Quantities: Reference Book (Energoatomizdat, Moscow, 1991) [in Russian].
M. Forsblom and G. Grimvall, “Anharmonic Effects in the Heat Capacity of Al,” Phys. Rev. B 69, 165106 (2004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Original Russian Text ©A.V. Fedorov, A.V. Shulgin.
Published in Fizika Goreniya i Vzryva, Vol. 51, No. 3, pp. 55–59, May–June, 2015.
Rights and permissions
About this article
Cite this article
Fedorov, A.V., Shulgin, A.V. Molecular dynamics modeling melting of of aluminum nanoparticles of the embedded atom method. Combust Explos Shock Waves 51, 333–337 (2015). https://doi.org/10.1134/S0010508215030089
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0010508215030089