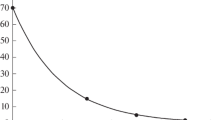
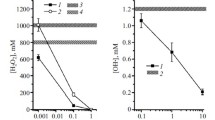
Abstract—This paper summarizes the relevant data on the use of chlorophyll derivatives as radioprotectors, which have been previously presented (partially and without analysis) in other works. We present and discuss findings from experiments that have shown an increase in the survival rate of chlorophyll-treated mice after γ-irradiation. Intramuscular injection of chlorophyll has been shown to lead to a pronounced decrease in leukopenia syndrome in irradiated animals. A reduction in malondialdehyde concentration in the blood and liver of irradiated animals treated with chlorophyll compared to the control group has been also found. These data suggest that suppression of the process of lipid peroxidation may be a molecular mechanism for the radioprotective effect of chlorophyll preparations. This has been confirmed by the experiments on registration of chemiluminescence accompanying lipid peroxidation in the presence of chlorophyllin (a water-soluble hydrolysis product of chlorophyll) at various concentrations. The effect of the studied drug has led to a decrease in the intensity of chemiluminescence, thus indicating a decrease in the intensity of the lipid oxidation under the action of free radicals in the sample.




Similar content being viewed by others
REFERENCES
I. M. Ivanov, A. S. Nikiforov, M. A. Yudin, et al., Radiats. Biol. Radioekol. 60, 175 (2020). https://doi.org/10.31857/S0869803120020058
S. Zimmering, O. Olvera, M. E. Hernandez, et al., Mutat. Res. 245, 47 (1990). https://doi.org/10.1016/0165-7992(90)90024-e
S. K. Abraham, L. Sarma, and P. C. Kesavan, Mutat. Res. 322, 209 (1994). https://doi.org/10.1016/0165-1218(94)90008-6
S. S. Kumar, B. Shankar, and K. B. Sainis, Biochim. Biophys. Acta 1672, 100 (2004). https://doi.org/10.1016/j.bbagen.2004.03.002
P. Morales-Ramirez and M. C. Garcia-Rodriguez, Mutat. Res. 320, 329 (1994). https://doi.org/10.1016/0165-1218(94)90085-x
P. Morales-Ramirez and M. T. Mendiola-Cruz, Mutat. Res. 344, 73 (1995). https://doi.org/10.1016/0165-1218(95)90041-1
A. V. Pozdeev, V. K. Promonenkov, N. P. Lysenko, Vet. Meditsina, No. 1, 42 (2010).
A. V. Pozdeev and V. P. Gugalo, Vestn. Kursk. Gos. S-kh. Akad., No. 2, 107 (2012).
A. V. Pozdeev, N. P. Lysenko, and V. N. Pozdeev, RF Patent 2508118, 2014.
A. V. Pozdeev, Vestn. Kursk. Gos. S-kh. Akad., No. 7, 53 (2013).
A. V. Pozdeev, Doctoral Dirrestation in Biology (Kostroma, 2015).
A. V. Pozdeev and N. P. Lysenko, Izv. Mezhd. Akad. Agrarn. Obraz. 42 (2), 60 (2018).
A. N. Grebenyuk, V. A. Basharin, R. A. Tarumov, et al., Vestn. Ross. Voen.-Med. Akad., No. 1 (41), 102 (2013).
M. v. Vasin, Radiats. Biol. Radioekol. 53, 459 (2013). https://doi.org/10.7868/S0869803113050160
B. N. Tarusov, in Initial Processes of Radiation Injury (Moscow, 1957), pp. 3–29.
A. M. Kuzin, Structural Metabolic Theory in Radiobiology (Nauka, Moscow, 1986) [in Russian].
L. F. Panchenko, A. I. Archakov, and T. A. Aleksandrova, Vopr. Med. Khimii, No. 5, 494 (1969).
V. P. Andreichuk, E. V. Andreichuk, N. P. Lysenko, and L. V. Rogozhina, RF Patent 2323733, 2008.
N. P. Lysenko, V. V. Pak, L. V. Rogozhina, and Z. G. Kusurova, Radiobiology: A Textbook, Ed. by N. P. Lysenko and V. V. Pak (Lan’, St Petersburg, 2019) [in Russian].
A. I. Zhuravlev and S. M. Zubkova, Antioxidants: Free Radical Pathology and Aging (Belye Al’vy, Moscow, 2014) [in Russian].
E. G. Khachaturov and V. V. Korobko, Byull. Bot. Sada Saratov. Gos. Univ. 17, 65 (2019). https://doi.org/10.18500/1682-1637-2019-1-65-72
T. M. Ong, W. Z. Whong, J. Stewart, and H. E. Brockman, Mutat. Res. 173, 111 (1986). https://doi.org/10.1016/0165-7992(86)90086-2
M. Ozcan, D. Aydemir, M. Bacanli, et al., Biol. Trace Elem. Res. 199, 4475 (2021). https://doi.org/10.1007/s12011-021-02585-6
L. A. Romodin, Izv. Saratov. Gos. Univ., Ser.: Khim. Biol. Ekol. 20, 427 (2020). https://doi.org/10.18500/1816-9775-2020-20-4-427-432
Yu. A. Vladimirov and E. V. Proskurnina, Usp. Biol. Khim. 49, 341 (2009).
A. I. Zhuravlev, Quantum Biophysics of Animals and Humans: A Textbook (Binom, Moscow, 2015) [in Russian].
V. A. Belyakov and R. F. Vassil’ev, Photochem. Photobiol. 11, 179 (1970). https://doi.org/10.1111/j.1751-1097.1970.tb05986.x
M. Pandurangan, H. Moorthy, and R. Sambandam, Cytotechnology 66, 839 (2014). https://doi.org/10.1007/s10616-013-9635-6
L. I. Wang, F. Liu, Y. Luo, et al., Biomed. Rep. 3, 425 (2015). https://doi.org/10.3892/br.2015.445
A. D. Belov and N. P. Lysenko, Radiats. Biol. Radioekol. 37, 772 (1997).
B. Halliwell and S. Chirico, Am. J. Clin. Nutr. 57, 715S (1993). https://doi.org/10.1093/ajcn/57.5.715S
M. Ruottinen, V. Kuosmanen, I. Saimanen, et al., Anticancer Res. 40, 253 (2020). https://doi.org/10.21873/anticanres.13947
E. B. Burlakova, M. V. Atkarskaya, L. D. Fatkullina, and S. G. Andreev, Radiats. Biol. Radioekol. 54, 162 (2014). https://doi.org/10.7868/S0869803114020040
X. Zhang, X. Xing, H. Liu, et al., Int. J. Radiat. Biol. 96 (5), 584 (2020). https://doi.org/10.1080/09553002.2020.1708993
M. Conrad and B. Proneth, Cell Res. 29, 263 (2019). https://doi.org/10.1038/s41422-019-0150-y
M. Daeihamed, S. Dadashzadeh, A. Haeri, and M. F. Akhlaghi, Curr. Drug Deliv. 14, 289 (2017). https://doi.org/10.2174/1567201813666160115125756
M. Arafat, C. Kirchhoefer, M. Mikov, et al., J. Pharm. Pharm. Sci. 20, 305 (2017). https://doi.org/10.18433/J3CK88
H. He, Y. Lu, J. Qi, et al., Acta Pharm. Sin. B 9, 36 (2019). https://doi.org/10.1016/j.apsb.2018.06.005
G. H. Naik, K. I. Priyadarsini, D. B. Naik, et al., Phytomedicine 11, 530 (2004). https://doi.org/10.1016/j.phymed.2003.08.001
L. P. Pashkova, A. V. Tsyganov, and N. P. Ponomarenko, Vopr. Normativno-Pravovogo Regulirovaniya v Veterinarii, No. 4, 186 (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies with the use of humans and animals as objects of research.
Additional information
Translated by A. Levina
Abbreviations: IL, interleukin.
Rights and permissions
About this article
Cite this article
Romodin, L.A., Lysenko, N.P. The Radioprotective Effect of Chlorophyll-Based Drugs. BIOPHYSICS 67, 78–84 (2022). https://doi.org/10.1134/S0006350922010158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350922010158