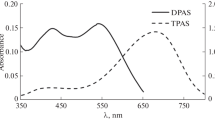
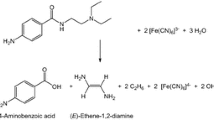
Abstract
A procedure for the direct photometric determination of rate constants for the oxidation of dithiothreitol (a thiol-compound example) by N-chlorotaurine and N-chloroglycine and their analogues that have a different structure of the reaction center was developed. The time dependences of the sum of the chloramine oxidant absorbance and the absorbance of dithiane (the product of the dithiothreitol conversion) were calculated for different values of the bimolecular rate constant. The value of the rate constant was considered as established when the best agreement between the calculated curve and the measured kinetic curve of absorbance was observed. The oxidative activities of monochloramine oxidants showed little difference: the rate constants for N-chlorotaurine, N-chloroglycine, and N-chloro-2,2-dimethyltaurine were equal to 170 ± 4, 235 ± 9.8, and 145 ± 4.3 M–1 s–1, respectively. When the reaction center structure was modified by introducing the substituents of the hydrogen atom into the chloramine group, the activities of the compounds changed dramatically: the rate constants for N-isopropyl-N-chlorotaurine, N,N-dichloro-2,2-dimethyltaurine and N-acetyl-N-chloro- 2,2-dimethyltaurine determined using the competitive kinetics method were 9 ± 0.5, 12 000 ± 950 and 25 300 ± 3000 M–1 s–1, respectively. The reactivity of N-chloroglycine and the structural analogues of taurine chloramine with respect to thiol-compounds correlates with the magnitude of the active chlorine charge. Predictions about the reactivity of unknown structural analogues of N-chloramino acids and N-chlorotaurine were obtained.











Similar content being viewed by others
REFERENCES
J. M. Zgliczynski, T. Stelmaszynska, J. Domanski, et al., Biochim. Biophys. Acta 235, 419 (1971).
E. L. Thomas, M. B. Grisham, and M. M. Jefferson, J. Clin. Invest. 72, 441 (1983).
C. C. Winterbourn, A. J. Kettle, and M. B. Hampton, Annu. Rev. Biochem. 85, 765 (2016).
M. A. Murina, D. I. Roshchupkin, N. N. Kravchenko, et al., Biophysics 42 (6), 1311 (1997).
T. W. Stief, J. Kurz, M. O. Doss, et al., Thromb. Res. 97, 473 (2000).
T. W. Stief, U. Feek, A. Ramaswamy, et al., Thromb. Res. 104, 361 (2001).
M. A. Murina, O. D. Fesenko, V. I. Sergienko, et al., Bull. Exp. Biol. Med. 134 (1), 36 (2002).
C. Martini, A. Hammer-Lrecher, M. Zuck, et al., Antimicrob. Agents Chemother. 56 (4), 1979 (2012).
J. Mustedanagic, V. F. Ximenes, and M. Nagl, AMB Expr. 7, 102 (2017).
M. Gruber, I. Moser, M. Nagl, et al., Antimicrob. Agents Chemother. 61 (5), e02527-16 (2017).https://doi.org/10.1128/AAC.02527-16 (2017).
S. A. Rani, C. Celeri, R. Najafi, et al., Urolithiasis 44 (3), 247 (2016).
W. Gottardi, M. Hagleitner, and M. Nagl, Arch. Pharm. Chem. Life Sci. 338 (10), 473 (2005).
L. Wang, B. Belisle, M. Bassiri, et al., Antimicrob. Agents Chemother. 55 (6), 2688 (2011).
A. C. Carr, C. L. Hawkins, S. R. Thomas, et al., Free Radic. Biol. Med. 30 (5), 526 (2001).
C. L. Hawkins, D. I. Pattison, and M. J. Davies, Amino Acids 25, 259 (2003).
A. V. Peskin and C. C. Winterbourn, Free Radic. Biol. Med. 30 (5), 572 (2001).
D. I. Roshchupkin, K. V. Kondrashova, and M. A. Murina, Biophysics 59 (6), 849 (2014).
W. Vogt, Free Radic. Biol. Med. 18 (1), 93 (1995).
W. Gottardi and M. Nagl, Arch. Pharm. Pharm. Med. Chem. 9, 411 (2002).
L. Wang, B. Khosrovi, and R. Najafi, Tetrahedron Lett. 49, 2193 (2008).
M. A. Murina, D. I. Roshchupkin, N. A. Chudina, et al., Bull. Exp. Biol. Med. 147 (6), 704 (2009).
D. I. Roshchupkin, M. A. Murina, and V. I. Sergienko, Biophysics 56 (5), 945 (2011).
D. I. Roshchupkin, M. A. Murina, N. N. Kravchenko, et al., Biofizika 52 (3), 527 (2007).
M. A. Murina, D. I. Roshchupkin, K. V. Kondrashova, et al., Bull. Exp. Biol. Med. 157 (2), 207 (2014).
D. I. Roshchupkin, K. V. Buravleva, M. A. Murina, et al., Biophysics 62 (1), 24 (2017).
D. Braghiroli and M. D. Bella, Tetrahedron Lett. 37, 7319 (1996).
K. S. Iyer and W. A. Klee, J. Biol. Chem. 248 (2), 707 (1973).
R. A. Johnson and F. D. Greene, J. Org. Chem. 40 (15), 2186 (1975).
P. Nagy and M. T. Ashby, J. Am. Chem. Soc. 129 (45), 14 082 (2007).
C. Storkey, M. J. Davies, and D. I. Pattison, Free Radic. Biol. Med. 73, 60 (2014).
D. Luo, S. W. Smith, and B. D. Anderson, J. Pharm. Sci. 94 (2), 304 (2005).
C. C. Winterbourn and D. Metodiewa, Free Radic. Biol. Med. 27 (3/4), 322 (1999).
H. Fukada and K. Takahashi, J. Biochem. 87 (4), 1105 (1980).
M.-H. Chau and J. W. Nelson, FEBS Lett. 291 (2), 296 (1991).
L. Carroll, D. I. Pattison, S. Fud, et al., Redox Biol. 12, 872 (2017).
W. A. Prutz, Arch. Biochem. Biophys. 371 (1), 107 (1999).
FUNDING
This work was performed with partial support from the Russian Foundation for Basic Research, project no. 16-04-00220.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by P. Kuchina
Rights and permissions
About this article
Cite this article
Roshchupkin, D.I., Sorokin, V.L., Semenkova, G.N. et al. The Properties of Biologically Significant Chloramine Oxidants: Reactivity and Its Dependence on the Structure of the Functional Atom Group. BIOPHYSICS 64, 145–154 (2019). https://doi.org/10.1134/S0006350919020155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350919020155