Abstract
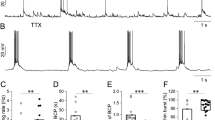
In this paper, the changes in the frequency and amplitude of action potentials (AP) were investigated depending on the depolarization caused by the Ca2+ channels activity during the spontaneous synchronous activity (SSA) of hippocampal neurons in culture. It is known that the pacemaker neuronal electrical activity can be both tonic and bursting. Using the image analysis to measure [Са2+]i and patch-clamp to register the membrane potential we show that depolarization caused by the GABA(A) receptor inhibitor results in a pattern of SSA in which tonic APs frequency of 2−3 Hz are generated by a neuron without any changes in cytosolic free Ca2+ concentration, ([Ca2+]i). The tonic mode is interrupted by bursts activity, which is accompanied by slow depolarization and calcium pulses. The inhibitor of T-type calcium channels, ML218, suppresses this process. The frequency and amplitude of the AP are regulated by slow depolarization pulses as follows: on the depolarization front, the APs frequency increases. At the same time, the amplitude decreases due to Na+ channels inactivation. The higher is the depolarization rate, the higher is the APs frequency. If slow depolarization amplitude exceeds Na+ channels reactivation potential, the neuronal impulse activity stops. As the [Ca2+]i increases and Ca2+-dependent K+ channels are activated, depolarization amplitude decreases slowly, and Na+ channels are reactivated, which leads to a gradual increase in the amplitude of APs against the background of depolarization decrease. The frequency of APs on the backside of depolarization pulse slows down to 3–4 Hz due to the occurrence of the faster Ca2+-potential oscillations (micro bursts of AP). Under these conditions, only one AP can be generated due to rapid depolarization at the leading edge. After that, APs generation is suppressed due to Na+ channel inactivation. The frequency of APs in this case coincides with the Ca2+ channel activation frequency (3−4 Hz). The burst firing is terminated due to [Ca2+]i increase, Ca2+-dependent K+ channels activation, and voltage-gated Ca2+ channels (VGCC) inactivation. As a result, the membrane is hyperpolarized even more (10 mV lower than the critical potential), that suppress AP generation, activate HCN-like channels and reactivate Na+ and VGCC. The activity of HCN-like channels increases, the membrane slowly depolarizes and reaches the threshold potential. The generation of tonic APs begins, and then, the Ca2+ channels opens, and Ca2+ potential and Ca2+ signal induced again. Thus, the Ca2+-channels, is determining the pulse of slow depolarization, control the frequency and the amplitude of APs during SSA, regulating the activation and inactivation conditions of Na+ channels. The T-type channels inhibitors reduce the duration of burst firing and Ca2+ impulse in neurons in vitro, stimulating their survival during hyperexcitation and ischemia. Thus, reducing the Ca2+ pulse duration caused by the inhibitors of T-type Ca2+-channels, can be one of the reasons for the known neuroprotective effect of these compounds.
Keywords: spontaneous synchronous activity of neurons, calcium signal, T-type calcium channels, voltage-gated calcium channels, action potential, genesis of bursting activity, action potential bursts, depolarization shift, critical potential





Similar content being viewed by others
REFERENCES
S. M. Lu, W. Guido, and S. M. Sherman, J. Neurosci. 68 (6), 2185 (1992).
S. Anava, A. Greenbaum, E. Ben Jacob, et al., Biophys. J. 96 (4), 1661 (2009).
A. V. Kononov, N. V. Ball, and V. P. Zinchenko, Biochemistry (Moscow). Suppl. Ser. A: Membr. Cell Biol. 5 (2), 162 (2011).
D. A. McCormick and D. Contreras, Annu. Rev. Physiol. 63, 815 (2001).
I. Nikonenko, M. Bancila, A. Bloc, et al., Mol. Pharmacol. 68 (1), 84 (2005).
B. J. Kopecky, R. Liang, and J. Bao, Pflügers Arch. 466 (4), 757 (2014).
B. Nilius, K. Tala, and A. Verkhratsky, Cell Calcium 40 (2), 81 (2006).
D. Kim, I. Song, S. Keum, et al., Neuron 31 (1), 35 (2001).
I. Song, D. Kim, S. Choi, et al., J. Neurosci. 24 (22), 5249 (2004).
S. Huc, A. Monteil, I. Bidaud, et al., Biochim. Biophys. Acta 1793 (6), 947 (2009).
E. Perez-Reyes, L. L. Cribbs, A. Daud, et al., Cell. Mol. Life Sci. 56 (7–8), 660 (1999).
M. Chevalier, P. Lory, C. Mironneau, et al., Eur. J. Neurosci. 23 (9), 2321 (2006).
J. Proft and N. G. Weiss, Mol. Pharmacol. 87 (6), 890 (2015).
E. Perez-Reyes, Mol. Pharmacol. 77 (2), 136 (2010).
J. L. Sánchez-Alonso, J. V. Halliwell, and A. Colino, Neurosci. Lett. 439 (3), 275 (2008).
R. Iyer, M. A. Ungless, and A. A. Faisa, Sci. Rep. 7 (1), 5248 (2017).
R. Rehak, T. M. Bartoletti, J. D. T. Engbers, et al., PloS One 8, e61844 (2013). doi 10.1371/journal.pone. 0061844
J. Wolfart and J. J. Roeper, Neuroscience 22 (9), 3404 (2002).
J. Xu and C. E. Clancy, PloS One 3 (4), e2056 (2008). doi 10.1371/journal.pone. 0002056
J. T. Wolfe, H. Wang, E. Perez-Reyes, and P. Q. Barrett, J. Physiol. 538 (2), 343 (2002).
J. Chemin, A. Mezghrani, I. Bidaud, et al., J. Biol. Chem. 282 (45), 32710 (2007).
J. Chemin, A. Monteil, E. Perez-Reyes, et al., EMBO J. 20 (24), 7033 (2001).
J. Chemin, J. Nargeot, and P. Lory, J. Biol. Chem. 282 (4), 2314 (2007).
K. Talavera, M. Staes, A. Janssens, et al., J. Gen. Physiol. 124 (3), 225. (2004).
Y. Zhang, L. L. Cribbs, and J. Satin, Am. J. Physiol. Heart Circ. Physiol. 278 (1), H184 (2000).
J. T. Wolfe, H. Wang, J. Howard, et al., Nature 424 (6945), 209 (2003).
J. Tao, M. E. Hildebrand, P. Liao, et al., Mol. Pharmacol. 73 (6), 1596 (2008).
M. E. Hildebrand, L. S. David, J. Hamid, et al., J. Biol. Chem. 282 (29), 21043 (2007).
M. T. Nelson, S. M. Todorovic, and E. Perez-Reyes, Curr. Pharmaceut. Design 12 (18), 2189 (2006).
S. M. Todorovic and V. Jevtovic-Todorovic, Pflügers Arch. 466 (4), 701 (2014).
B. J. Kopecky, R. Liang, and J. Bao, Eur. J. Physiol. 466 (4), 757 (2014).
P. Orestes, D. Bojadzic, R. M. Chow, and S. M. Todorovic, Mol. Pharmacol. 75 (3), 542 (2009).
N. C. Spitzer and E. X. Olson, J. Neurobiol. 26 (3), 316 (1995).
H. P. Robinson, M. Kawahara, et al., J. Neurophysiol. 70 (4), 1606 (1993).
J. N. Guzman, J. Sánchez-Padilla, C. S. Chan, and D. J. Surmeier, J. Neurosci. 29 (35), 11011 (2009).
D. J. Surmeier and P. T. Schumacker, J. Biol. Chem. 288 (15), 10736 (2013).
O. J. Lieberman, S. J. Choi, E. Kanter, et al., eNeuro. 4 (6), 0167 (2017).
D. J. Surmeier, J. A. Obeso, and G. M. Halliday, Nat. Rev. Neurosci. 18 (2), 101 (2017).
C. S. Chan, J.N. Guzman, E. Ilijic, et al., Nature 447 (7148), 1081 (2007).
G. Grynkiewicz, M. Poenie, and R. Y. Tsien, J. Biol. Chem. 260 (6), 3440 (1985).
H. Hayashi, H. Miyata, H. Terada, et al., Jpn. Heart J. 35 (5), 673 (1994).
L. Cozzi, P. D’Angelo, and V. Sanguineti, Biol. Cybern. 94 (5), 335 (2006).
S. M. Cain and T. P. Snutch, Biochim. Biophys. Acta 1828 (7), 1572 (2013).
M. V. Turovskaya, E. A. Turovsky, V. P. Zinchenko, et al., Neurosci. Lett. 516 (1), 151 (2012).
E. A. Turovsky, M. V. Turovskaya, S. G. Gaidin, and V. P. Zinchenko, Arch. Biochem. Biophys. 615, 35 (2017).
E. A. Turovsky, V. P. Zinchenko, S. G. Gaidin, and M. V. Turovskaya, Biochemistry (Moscow). Suppl. Ser. A: Membr. Cell Biol. 12, 74 (2018).
V. P. Zinchenko, M. V. Turovskaya, I. Yu. Teplov, et al., Biophysics (Moscow) 61 (1), 85 (2016).
E. M. Izhikevich, Int. J. Bifurcat. Chaos 10 (6), 1171 (2000).
H. P. Robinson, K. Torimitsu, Y. Jimbo, et al., Jpn. J. Physiol. 43 (1), 125 (1993).
V. V. Dynnik, A. V. Kononov, A. V. Sergeev, I. Yu. Tep-lov, V. P. Zinchenko, Plos One. 10 (7) e0134145. doi: 10.1371/journal.pone.0134145 (2015)
D. Cooper, Neurochem. Int. 41 (5), 333 (2002).
H. A. Swadlow, A. G. Gusev, and T. Bezdudnaya, J. Neurosci. 22 (17), 7766 (2002).
V. P. Zinchenko, E. A. Turovsky, M. V. Turovskaya, et al., Biochemistry (Moscow). Suppl. Ser. A: Membr. Cell Biol. 10 (2), 118 (2016).
V. P. Zinchenko, S. G. Gaidin, I. Y. Teplov, and A. M. Kosenkov, Biochemistry (Moscow). Suppl. Ser. A: Membr. Cell Biol. 11 (4), 261 (2017).
ACKNOWLEDGMENTS
This work was supported by the Committee of Science of MES RK, grant AP05133528.
Author information
Authors and Affiliations
Corresponding author
Additional information
1The article was translated by the authors.
Abbreviations: SSA – spontaneous synchronous activity, Ca2+ signal, T-type Ca2+ channels, VGCC – voltage-gated calcium channels, AP – action potential, genesis of bursting activity, action potential burst, depolarization shift, critical potential, [Ca2+]i – cytoplasmic Ca2+ concentration.
Rights and permissions
About this article
Cite this article
Teplov, I.Y., Tuleukhanov, S.T. & Zinchenko, V.P. Regulation of Action Potential Frequency and Amplitude by T-type Ca2+ Channel During Spontaneous Synchronous Activity of Hippocampal Neurons. BIOPHYSICS 63, 566–575 (2018). https://doi.org/10.1134/S0006350918040206
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350918040206