Abstract
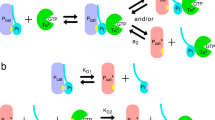
The kinetic behavior of cGMP-specific phosphodiesterase in a totally bleached bovine retinal rod outer segment suspension was studied by the pH-metric method at high and low concentrations of free calcium ions (≈100 μM and <10 nM, respectively). The phosphodiesterase was activated by low GTP concentrations (∼1–2 μM) that were comparable with the concentration of G-protein transducin, the GTP-binding alpha-subunit of which is the intrinsic activator of photoreceptor phosphodiesterase. The results allow the suggestion that, besides the earlier described system of RGS proteins participating in acceleration of GTP hydrolysis, rod outer segments also contain an additional Ca2+-dependent mechanism to inactivate so-called “free” transducin, i.e. active transducin that has not managed to interact with phosphodiesterase within the time restricted by the duration of photoreceptor response.
Similar content being viewed by others
References
L. Stryer, Annu. Rev. Neurosci. 9, 87 (1986).
L. Stryer, J. Biol. Chem. 266, 10711 (1991).
V. Y. Arshavsky, T. D. Lamb, and E. N. Pugh, Jr., Annu. Rev. Physiol. 64, 153 (2002).
E. N. Pugh Jr. and T. D. Lamb, Biochim. Biophys. Acta 1141(2–3), 111 (1993).
E. E. Fesenko, S. S. Kolesnikov, and A. L. Lyubarsky, Nature 313, 310 (1985).
K.-W. Yau, and K. Nakatani, Nature 309, 352 (1984).
A. L. Hodgkin, P. A. McNaughton, and B. J. Nunn, J. Physiol. 358, 447 (1985).
K.-W. Yau and K. Nakatani, Nature 311, 661 (1984).
A. L. Hodgkin, P. A. McNaughton, and B. J. Nunn, J. Physiol. 391, 347 (1987).
L. Cervetto, L. Lagnado, R. J. Perry, et al., Nature 337, 740 (1989).
K.-W. Yau and K. Nakatani, Nature 313, 579 (1985).
P. A. McNaughton, L. Cervetto, and B. J. Nunn, Nature 322, 261 (1986).
E. N. Pugh Jr. and T. D. Lamb, in Handbook of Biological Physics, Ed. by D. G. Stavenga, E. N. Pugh Jr., and W. J. de Grip (Elsevier Science B.V., Amsterdam, 2000), Chapter 5, 183.
M. E. Burns and E. N. Pugh Jr., Physiology (Bethesda) 25, 72 (2010).
M. E. Burns and V. Y. Arshavsky, Neuron 48, 387 (2005).
V. Yu. Arshavsky, M. P. Gray-Keller, and M. D. Bownds, J. Biol. Chem. 266, 18530 (1991).
D. A. Baylor, T. D. Lamb, and K.-W. Yau, J. Physiol. 288, 613 (1979).
J. K. Angleson and T. G. Wensel, Neuron 11, 939 (1993).
J. K. Angleson, and T. G. Wensel, J. Biol. Chem. 269, 16290 (1994).
W. He, C. W. Cowan, and T. G. Wensel, Neuron 20, 95 (1998).
W. He, L. Lu, X. Zhang, et al., J. Biol. Chem. 275, 37093 (2000).
C. W. Cowan, W. He, and T. G. Wensel, Prog. Nucleic Acid Res. Mol. Biol. 65, 341 (2000).
C. W. Cowan, T. G. Wensel, and V. Y. Arshavsky, Methods Enzymol. 315, 524 (2000).
V. Z. Slepak, Prog. Mol. Biol. Transl. Sci. 86, 157 (2009).
C. W. Cowan, R. N. Fariss, I. Sokal, et al., Proc. Natl. Acad. Sci. USA 95, 5351 (1998).
N. Ya. Orlov, E. V. Kalinin, T. G. Orlova, and et al., Biochim. Biophys. Acta 954, 325 (1988).
V. G. Tishchenkov, Candidate’s Dissertation in Biology (Minsk, 1883).
R. Yee and P. A. Liebman, J. Biol. Chem. 253, 8902 (1978).
P. A. Liebman and A. T. Evanczuk, Methods Enzymol. 81, 532 (1982).
M. M. Bradford, Anal. Biochem. 72, 248 (1976).
N. Ya. Orlov, Systems of Phototransduction of Vertebrate Retinal Rods and Cones. Molecular Mechanisms (LAP LAMBERT Academic Publishing GmbH & Co KG, Saarbrücken, Deutschland, 2011).
H. Kuhn, Nature 283, 587 (1980).
N. Ya. Orlov, A. A. Freidin, D. N. Orlov, et al., Abstr. Internat. Conf. “Reception and Intracellular Signaling” (Izd. IBK RAN, Pushchino, 1998), pp. 108–110.
K.-W. Koch, J. Biol. Chem. 266, 8634 (1991).
K. Palczewski, J. H. McDowell, and P. A. Hargrave, J. Biol. Chem. 263, 14067 (1988).
U. Laitko and K. P. Hofmann, Biophys. J. 74, 803 (1998).
K.-W. Koch and L. Stryer, Nature 334, 64 (1988).
K.-W. Koch, F. Eckstein, and L. Stryer, J. Biol. Chem. 265, 9659 (1990).
K. Palczewski, J. H. McDowell, and P. A. Hargrave, J. Biol. Chem. 263, 14067 (1988).
J. Buczy ko, C. Gutmann, and K. Palczewski, Proc. Natl. Acad. Sci. USA 88, 2568 (1991).
L. A. Astakhova, M. L. Firsov, and V. I. Govardovskii, J. Gen. Physiol. 132, 587 (2008).
M. L. Firsov, Doctoral Dissertation in Biology (St. Petersburg, 2012).
V. M. Grishchenko, T. G. Orlova, A. A. Freidin, et al., Dokl. AN SSSR 307, 1498 (1989).
A. M. Dizhur, E. N. Nekrasova, and P. P. Filippov, Biokhimiia (Moscow) 56, 225 (1991a).
A. M. Dizhoor, S. Ray, S. Kumar, S., et al., Science 251, 915 (1991).
S. Kawamura, Nature 362, 855 (1993).
S. Kawamura, O. Hisatomi, S. Kayada, et al., J. Biol. Chem. 268, 14579 (1993).
W. A. Gorczyca, M. P. Gray-Keller, P. B. Detwiler, and K. Palczewski, Proc. Natl. Acad. Sci. USA 91, 4014 (1994).
A. M. Dizhoor, D. G. Lowe, E. V. Olshevskaya, et al., Neuron 12, 1345 (1994).
A. M. Dizhoor, E. M. Olshevskaya, W. J. Henzel, et al., J. Biol. Chem. 270, 25200 (1995).
M. A. Kutuzov and N. Bennett, Eur. J. Biochem. 238, 613 (1996)
H. Ohguro, M. Rudnicka-Nawrot, J. Buczylko, et al., J. Biol. Chem. 271, 5215 (1996).
G. Hu, G.-F. Jang, C.W. Cowan, et al., J. Biol. Chem. 276, 22287 (2001).
T. G. Wensel, Adv. Exp. Med. Biol. 514, 125 (2002).
I. Sokal, G. Hu, Y. Liang, et al., J. Biol. Chem. 278, 8316 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Petrukhin, T.G. Orlova, A.R. Nezvetsky, N.Ya. Orlov, 2014, published in Biofizika, 2014, Vol. 59, No. 5, pp. 854–861.
Rights and permissions
About this article
Cite this article
Petrukhin, O.V., Orlova, T.G., Nezvetsky, A.R. et al. Activation of bovine retinal rod outer segment cGMP-specific phosphodiesterase by the transducin-GTP complex in a physiologically significant range of free calcium ion concentrations. BIOPHYSICS 59, 694–699 (2014). https://doi.org/10.1134/S0006350914050200
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350914050200