Abstract
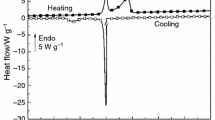
The data on the effect of temperature on the kinetics of collagen fibril formation at physiological pH values and ionic strength in the temperature range 26–39°C have been analyzed. The temperature of 35°C optimal for collagen fibril formation has been defined as the point of inflection for half-maximal turbidity and collagen molecule microunfolding values, which corresponds to the temperature of the first transition on the heat absorption curve. The temperature range (32–35°C) in which collagen microunfolding stimulates fibril formation has been determined.
Similar content being viewed by others
References
A. Veis and K. Payne, in Collagen, Ed. by M. Nimni (CRC Press, Boca Raton, 1988), Vol. 1, pp. 113–137.
N. G. Esipova, Biofizika 2, 461 (1957).
N. G. Esipova, M. V. Grigolava, T. Yu. Shchegoleva, et al., Biofizika 26, 355 (1981).
V. Ya. Aleksandrov, Cells, Macromolecules and Temperature (Nauka, Leningrad, 1975) [in Russian].
T. V. Burdzhanadze, E. I. Tiktopulo, and P. L. Privalov, Dokl. AN SSSR 293, 720 (1987).
N. G. Esipova, N. S. Andreeva, and T. V. Gatovskaya, Biofizika 3, 529 (1958).
W. V. Arnold, A. Fertala, A. L. Sieron et al., J. Biol. Chem. 273, 31822 (1998).
J. M. Davis and H. P. Bachinger, J. Biol. Chem. 268, 25965 (1993).
A. Veis and A. George, Extracellular Matrix Assembly and Structure, Ed. by Yurchenco et al. (Academic Press, New York, 1994), pp. 15–45.
K. E. Kadler, Y. Hojima, and J. Prockop., J. Biol. Chem. 263, 10517 (1988).
T. I. Nikolaeva, E. I. Tiktopulo, R. V. Polozov, and Yu. A. Rochev, Biofizika 52, 261 (2007).
P. L. Privalov and E. I. Tiktopulo, Biopolymers 9, 127 (1970).
C. M. Stoscheck, Meth. Enzym. 182, 50 (1990).
F. H. Silver and D. E. Birk. Coll. Rel. Res. 3, 393 (1983).
P. L. Privalov, E. I. Tiktopulo, and V. M. Tischenko, J. Mol. Biol. 127, 203 (1979).
E. Leikina, M. V. Mertts, N. Kuznetsova, and S. Leikin, Proc. Natl. Acad. Sci. USA 99, 1314 (2002).
Author information
Authors and Affiliations
Additional information
Original Russian Text © T.I. Nikolaeva, S.M. Kuznetsova, E.I. Tiktopulo, 2009, published in Biofizika, 2009, Vol. 54, No. 6, pp. 1015–1018.
Rights and permissions
About this article
Cite this article
Nikolaeva, T.I., Kuznetsova, S.M. & Tiktopulo, E.I. Does collagen microunfolding stimulate fibril formation?. BIOPHYSICS 54, 683–685 (2009). https://doi.org/10.1134/S0006350909060049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350909060049