Abstract
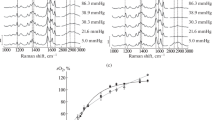
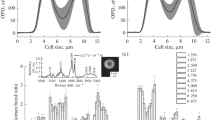
The dimensions and refractive properties of erythrocytes and the affinity of their hemoglobin for oxygen have been assessed using laser interference microscopy and Raman spectroscopy. Lowering the pH and addition of 2,3-diphosphoglycerate cause comparable changes in the cell shape and the state of cytoplasm, as well as in the oxygen affinity of hemoporphyrin, but the mechanisms of these effects appear to be different. It is shown that acidification mainly decreases the hemoporphyrin oxygen-binding capacity, whereas 2,3-diphosphoglycerate increases the ability of hemoporphyrin to release oxygen.
Similar content being viewed by others
Abbreviations
- CRI:
-
cytoplasm refractive index
- DPG:
-
2,3-diphosphoglycerate
- Hb:
-
hemoglobin
- LIM:
-
laser interference microscopy
- RS:
-
Raman scattering
References
T. H. Han, E. Qamirani, A. G. Nelson, et al., Proc. Natl. Acad. Sci. USA 100, 12504 (2003).
F. B. Jensen, Acta Physiol. Scand. 182, 215 (2004).
C. Lenfant, J. Torrance, E. English, et al., J. Clin. Invest. 47, 2652 (1968).
B. D. Smith and P. L. La Celle, Blood 53, 15 (1979).
J. A. Walder, R. Chatterjee, T. L. Steck, et al., J. Biol. Chem. 259, 10238 (1984).
R. E. Weber, W. Voelter, A. Fago, et al., Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R454 (2004).
O. V. Rodnenkov, O. G. Luneva, N. A. Ulyanova, et al., Pathophysiology 11, 209 (2005).
O. G. Luneva, N. A. Brazhe, O. E. Fadyukova, et al., Dokl. RAN 405, 834 (2005).
P. Cary, Use of RS and RRS Spectroscopy in Biochemistry (Mir, Moscow, 1985).
K. N. Solov’ev, L. L. Gladkov, A. S. Starukhin, and S. F. Shkirman, Porphyrin Spectroscopy: Vibrational States (Nauka i Tekhnika, Minsk, 1985) [in Russian].
D. D. Protsenko, O. A. Dyagileva, Yu. K. Novoderzhkina, et al., Atlas of Blood and Bone Marrow Cells (Moscow, 2004) [in Russian].
S. Tang, Proc. SPIE 2860, 34 (1996).
G. G. Levin, F. V. Bulygin, and G. N. Vishnyakov, Tsitologiya 47, 348 (2005).
A. I. Yusipovich, S. M. Novikov, T. A. Kazakova, et al., Kvant. Elektron. 36, 874 (2006).
M. Bessis, Corpuscles: Atlas of Red Blood Cell Shape (1974).
P. Mazeron, J. Didelon, S. Muller, and J. F. Stoltz, Photochem. Photobiol. 72, 172 (2000).
S. F. Pedersen, E. K. Hoffmann, and J. W. Mills, Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 385 (2001).
N. N. Barvitenko, N. C. Adragna, and R. E. Weber, Cell Physiol. Biochem. 15, 1 (2005).
M. P. Sheetz and J. Casaly, J. Biol. Chem. 255, 9955 (1980).
S. E. Lux, Semin. Hematol. 16, 21 (1979).
J. Duhm, Pflugers Arch. 363, 55 (1976).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.Yu. Bryzgalova, N.A. Brazhe, A.I. Yusipovich, G.V. Maksimov, A.B. Rubin, 2009, published in Biofizika, 2009, Vol. 54, No. 3, pp. 442–447.
Rights and permissions
About this article
Cite this article
Bryzgalova, N.Y., Brazhe, N.A., Yusipovich, A.I. et al. Role of the state of erythrocyte cytoplasm in the change of hemoglobin affinity for oxygen. BIOPHYSICS 54, 308–311 (2009). https://doi.org/10.1134/S0006350909030075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350909030075