Abstract
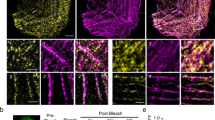
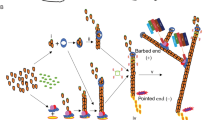
The effect of the suppression of expression of the actin-binding protein caldesmon on the motility of nonmuscle cells has been studied. A more than a fivefold decrease in the content of this protein in cells by RNA interference led to the disturbance of the formation of actin stress fibers and acceleration of cell migration to the zone of injury of the monolayer. A stimulation of stationary cells by serum induced more than 1,5-fold accumulation of stress fibers only in control cells, but not in caldesmon-deficient cells. Similarly, the accumulation of actin filaments was observed in actively migrating cells of only wild type, but not in the cells with low caldesmon content. These changes occurred mainly at the leading edge of the migrating cell where the distinct structure of actin filaments was not seen in the absence of caldesmon. It was assumed that caldesmon inhibits cell migration due to the stabilization of actin in filaments and a decrease in the dynamics of monomeric actin at the leading edge of the migrating cell.
Similar content being viewed by others
Abbreviations
- FCS:
-
fetal calf serum
- shRNA:
-
short hairpin RNA
- GFP:
-
green fluorescent protein
- PBS:
-
phosphate buffered saline
- CPFB:
-
cytoskeleton protein fixation buffer
- GAPDH:
-
glyceraldehyde phosphate dehydrogenase
References
A. J. Ridley, M. A. Schwartz, E. Burridge, et al., Science 302(5651), 1704 (2003).
D. A. Lauffenburger and A. F. Horwitz, Cell 84(3), 359 (1996).
J. A. Theriot and T. J. Mitchison, Nature 352(6331), 126 (1991).
L. P. Cramer, T. J. Mitchison, and J. A. Theriot, Curr. Opin. Cell. Biol. 6(1), 82 (1994).
J. V. Small and G. P. Resch, Curr. Opin. Cell. Biol. 17(5), 517 (2005).
T. J. Mitchison and L. P. Cramer, Cell 84(3), 371 (1996).
L. M. Machesky and K. L. Gould, Curr. Opin. Cell. Biol. 11(1), 117 (1999).
A. Bershadsky, Trends Cell. Biol. 14(11), 589 (2004).
T. D. Pollard and G. G. Borisy, Cell 112(4), 453 (2003).
S. J. Winder and K. P. Ayscough, J. Cell. Sci. 118(4), 651 (2005).
A. V. Vorotnikov, M. A. Krimskiy and V. P. Shirinskiy, Biochem. 67, 1587 (2002).
M. J. Greenberg, C. L. Wang, W. Lehman, and J. R. Moore, Cell. Motil. Cytoskeleton 65(2), 156 (2008).
R. Dabrowska, N. Kulikova, and M. Gagola, Protoplasma 224(1–2), 1 (2004).
J. Kordowska, R. Huang, and C. L. A. Wang, J. Biomed. Sci. 13(2), 159 (2006).
D. M. Helfman, E. T. Levy, C. Berthier, et al., Mol. Biol. Cell 10(10), 3097 (1999).
R. Eves, B. A. Webb, S. Zhou, and A. S. Mak, J. Cell. Sci. 119(9), 1691 (2006).
T. Mirzapoiazova, I. A. Kolosova, L. Romer, et al., J. Cell. Physiol. 203(3), 520 (2005).
K. S. Warren, D. C. Shutt, J. P. McDermott, et al., Cell. Motil. Cytoskeleton. 34(3), 215 (1996).
R. Ishikawa, S. Yamashiro, and F. Matsumura, J. Biol. Chem. 264(13), 7490 (1989).
R. Ishikawa, S. Yamashiro, K. Kohama, and F. Matsumura, J. Biol. Chem. 273(41), 26991 (1998).
Y. Yamakita, F. Oosawa, S. Yamashiro, and F. Matsumura, J. Biol. Chem. 278(20), 17937 (2003).
V. B. Patchell, A. V. Vorotnikov, Y. Gao, et al., Biochim. Biophys. Acta 1596(1), 121 (2002).
R. Huang, L. Li, H. Guo, and C. L. Wang, Biochemistry 42(9), 2513 (2003).
E. A. Goncharova, V. P. Shirinsky, A. Y. Shevelev, et al., FEBS Lett. 497(2–3), 113 (2001).
E. A. Goncharova, A. V. Vorotnikov, E. 0. Gracheva, et al., Biol. Chem. 383(1), 115 (2002).
Z. Gu, J. Kordowska, G. L. Williams, et al., Exp. Cell. Res. 313(5), 849 (2007).
R. D. Eppinga, Y. Li, J. L. Lin, et al., Cell. Motil. Cytoskeleton 63(9), 543 (2006).
U. K. Laemmli, Nature 227(5259), 680 (1970).
W. Schaffner and C. Weissmann, Anal. Biochem. 56(2), 502 (1973).
H. Towbin, T. Staehelin, and J. Gordon, Proc. Natl. Acad. Sci. USA 76(9), 4350 (1979).
K. G. Birukov, V. P. Shirinsky, A. V. Vorotnikov, and N. B. Gusev, FEBS Lett. 262(2), 263 (1990).
T. Morita, T. Mayanagi, T. Yoshio, and K. Sobue, J. Biol. Chem. 282(11), 8454 (2007).
B. T. Gabelt, Y. Hu, and J. L. Vittitow, Exp. Eye Res. 82(6), 935 (2006).
A. D. Bershadsky, N. Q. Balaban, and B. Geiger, Annu. Rev. Cell Dev. Biol. 19, 677 (2003).
J. Kordowska, T. Hetrick, L. P. Adam, and C. L. A. Wang, Exp. Cell Res. 312(2), 95 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.V. Kudryashova, P.N. Rutkevich, A.Ya. Shevelev, T.N. Vlasik, A.V. Vorotnikov, 2008, published in Biofizika, 2008, Vol. 53, No. 6, pp. 978–985.
Rights and permissions
About this article
Cite this article
Kudryashova, T.V., Rutkevich, P.N., Shevelev, A.Y. et al. Caldesmon affects actin organization at the leading edge and inhibits cell migration. BIOPHYSICS 53, 527–532 (2008). https://doi.org/10.1134/S0006350908060110
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350908060110