Abstract
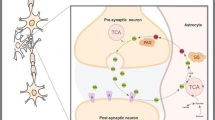
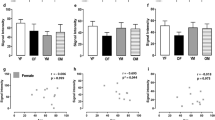
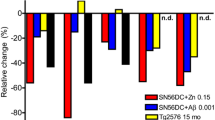
GABA and glutamate are the most abundant neurotransmitters in the CNS and play a pivotal part in synaptic stability/plasticity. Glutamate and GABA homeostasis is important for healthy aging and reducing the risk of various neurological diseases, while long-term imbalance can contribute to the development of neurodegenerative disorders, including Alzheimer’s disease (AD). Normalization of the homeostasis has been discussed as a promising strategy for prevention and/or treatment of AD, however, data on the changes in the GABAergic and glutamatergic systems with age, as well as on the dynamics of AD development, are limited. It is not clear whether imbalance of the excitatory/inhibitory systems is the cause or the consequence of the disease development. Here we analyzed the age-related alterations of the levels of glutamate, GABA, as well as enzymes that synthesize them (glutaminase, glutamine synthetase, GABA-T, and GAD67), transporters (GLAST, GLT-1, and GAT1), and relevant receptors (GluA1, NMDAR1, NMDA2B, and GABAAr1) in the whole hippocampus of the Wistar rats and of the senescence-accelerated OXYS rats, a model of the most common (> 95%) sporadic AD. Our results suggest that there is a decline in glutamate and GABA signaling with age in hippocampus of the both rat strains. However, we have not identified significant changes or compensatory enhancements in this system in the hippocampus of OXYS rats during the development of neurodegenerative processes that are characteristic of AD.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- GABA:
-
gamma aminobutyric acid
- GABAAR1:
-
GABA-A receptor subunit 1
- GABA-T:
-
GABA transaminase
- GAD:
-
glutamic acid decarboxylase (glutamate decarboxylase)
- GAD67:
-
glutamic acid decarboxylase isoform
- GAT1:
-
type 1 GABA transporter
- GLAST:
-
glial glutamate and aspartate transporter
- GLT-1:
-
glial glutamate transporter 1
- GluA1:
-
AMPA receptor subunit 1
- NMDAR1:
-
NMDA receptor subunit 1
- NMDAR2B:
-
NMDA receptor subunit 2B
References
Wang, R., and Reddy, P. H. (2017) Role of glutamate and NMDA receptors in Alzheimer’s disease, J. Alzheimers Dis., 57, 1041-1048, https://doi.org/10.3233/JAD-160763.
Selkoe, D. J., and Hardy, J. (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years, EMBO Mol. Med., 8, 595-608, https://doi.org/10.15252/emmm.201606210.
Wyss-Coray, T. (2016) Ageing, neurodegeneration and brain rejuvenation, Nature, 539, 180-186, https://doi.org/10.1038/nature20411.
Polanco, J. C., Li, C., Bodea, L. G., Martinez-Marmol, R., Meunier, F. A., and Götz, J. (2017) Amyloid-β and tau complexity – towards improved biomarkers and targeted therapies, Nat. Rev. Neurol., 14, 22-39, https://doi.org/10.1038/nrneurol.2017.162.
Cox, M. F., Hascup, E. R., Bartke, A., and Hascup, K. N. (2022) Friend or foe? Defining the role of glutamate in aging and Alzheimer’s disease, Front. Aging, 3, 929474, https://doi.org/10.3389/fragi.2022.929474.
Hampe, C. S., Mitoma, H., and Manto, M. (2018) GABA and glutamate: their transmitter role in the CNS and pancreatic islets, in GABA and Glutamate – New Developments in Neurotransmission Research, IntechOpen, Book Chapter 5, pp. 65-90, https://doi.org/10.5772/intechopen.70958.
Sears, S. M. S., and Hewett, S. J. (2021) Influence of glutamate and GABA transport on brain excitatory/inhibitory balance, Exp. Biol. Med. (Maywood), 246, 1069-1083, https://doi.org/10.1177/1535370221989263.
Kolosova, N. G., Stefanova, N. A., Korbolina, E. E., Fursova, A. Z., and Kozhevnikova, O. S. (2014) Senescence-accelerated OXYS rats: a genetic model of premature aging and age-related diseases, Adv. Gerontol., 27, 336-340, https://doi.org/10.1134/S2079057014040146.
Stefanova, N. A., Muraleva, N. A., Korbolina, E. E., Kiseleva, E., Maksimova, K. Y., and Kolosova, N. G. (2015) Amyloid accumulation is a late event in sporadic Alzheimer’s disease-like pathology in nontransgenic rats, Oncotarget, 6, 1396-1413, https://doi.org/10.18632/ONCOTARGET.2751.
Kolosova, N. G., Kozhevnikova, O. S., Muraleva, N. A., Rudnitskaya, E. A., Rumyantseva, Y. V., Stefanova, N. A., Telegina, D. V., Tyumentsev, M. A., Fursova, A. Z. (2022) SkQ1 as a tool for controlling accelerated senescence program: experiments with OXYS rats, Biochemistry (Moscow), 87, 1552-1562, https://doi.org/10.1134/S0006297922120124.
Stefanova, N. A., Maksimova, K. Y., Kiseleva, E., Rudnitskaya, E. A., Muraleva, N. A., and Kolosova, N. G. (2015) Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression of Alzheimer’s disease-like pathology, J. Pineal. Res., 59, 163-177, https://doi.org/10.1111/JPI.12248.
Telegina, D. V., Antonenko, A. K., Fursova, A. Z., and Kolosova, N. G. (2022) The glutamate/GABA System in the retina of male rats: effects of aging, neurodegeneration, and supplementation with melatonin and antioxidant SkQ1, Biogerontology, 23, 571-585, https://doi.org/10.1007/s10522-022-09983-w.
Stefanova, N. A., and Kolosova, N. G. (2023) The rat brain transcriptome: from infancy to aging and sporadic Alzheimer’s disease-like pathology, Int. J. Mol. Sci., 24, 1462, https://doi.org/10.3390/ijms24021462.
Stefanova, N. A., Maksimova, K. Y., Rudnitskaya, E. A., Muraleva, N. A., and Kolosova, N. G. (2018) Association of cerebrovascular dysfunction with the development of Alzheimer’s disease-like pathology in OXYS rats, BMC Genomics, 19, 51-63, https://doi.org/10.1186/S12864-018-4480-9.
Magi, S., Piccirillo, S., Amoroso, S., and Lariccia, V. (2019) Excitatory amino acid transporters (EAATs): glutamate transport and beyond, Int. J. Mol. Sci., 20, 5674, https://doi.org/10.3390/ijms20225674.
Pajarillo, E., Rizor, A., Lee, J., Aschner, M., Lee, E. (2019) The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics, Neuropharmacology, 161, 1-37, https://doi.org/10.1016/j.neuropharm.2019.03.002.
Snytnikova, O., Telegina, D., Savina, E., Tsentalovich, Y., and Kolosova, N. (2023) Quantitative metabolomic analysis of the rat hippocampus: effects of age and of the development of Alzheimer’s disease-like pathology, J. Alzheimer's Disease, https://doi.org/10.3233/JAD-230706, in press.
Schubert, F., Gallinat, J., Seifert, F., and Rinneberg, H. (2004) Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla, NeuroImage, 21, 1762-1771, https://doi.org/10.1016/j.neuroimage.2003.11.014.
Kaiser, L. G., Schuff, N., Cashdollar, N., and Weiner, M. W. (2005) Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T, Neurobiol. Aging, 26, 665-672, https://doi.org/10.1016/j.neurobiolaging.2004.07.001.
Chang, L., Jiang, C. S., and Ernst, T. (2009) Effects of age and sex on brain glutamate and other metabolites, Magn. Reson. Imaging, 27, 142-145, https://doi.org/10.1016/j.mri.2008.06.002.21.
Huang, D., Liu, D., Yin, J., Qian, T., Shrestha, S., and Ni, H. (2017) Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment, Eur. Radiol., 27, 2698-2705, https://doi.org/10.1007/S00330-016-4669-8.
Rozycka, A., Charzynska, A., Misiewicz, Z., Maciej Stepniewski, T., Sobolewska, A., Kossut, M., and Liguz-Lecznar, M. (2019) Glutamate, GABA, and presynaptic markers involved in neurotransmission are differently affected by age in distinct mouse brain regions, ACS Chem. Neurosci., 10, 4449-4461, https://doi.org/10.1021/acschemneuro.9b00220.
Segovia, G., Porras, A., Del Arco, A., and Mora, F. (2001) Glutamatergic neurotransmission in aging: a critical perspective, Mech. Ageing Dev., 122, 1-29, https://doi.org/10.1016/S0047-6374(00)00225-6.
Dong, Y., and Brewer, G. J. (2019) Global metabolic shifts in age and Alzheimer’s disease mouse brains pivot at NAD+/NADH redox sites, J. Alzheimers Dis., 71, 119-140, https://doi.org/10.3233/JAD-190408.
Mira, R. G., and Cerpa, W. (2020) Building a bridge between NMDAR-mediated\excitotoxicity and mitochondrial dysfunction in chronic and acute diseases, Cell. Mol. Neurobiol., 41, 1413-1430, https://doi.org/10.1007/S10571-020-00924-0.
Rodríguez-Giraldo, M., González-Reyes, R. E., Ramírez-Guerrero, S., Bonilla-Trilleras, C. E., Guardo-Maya, S., and Nava-Mesa, M. O. (2022) Astrocytes as a therapeutic target in Alzheimer’s disease-comprehensive review and recent developments, Int. J. Mol. Sci., 23, 13630, https://doi.org/10.3390/ijms232113630.
Yeung, J. H. Y., Palpagama, T. H., Wood, O. W. G., Turner, C., Waldvogel, H. J., Faull, R. L. M., and Kwakowsky, A. (2021) EAAT2 expression in the hippocampus, subiculum, entorhinal cortex and superior temporal gyrus in Alzheimer’s disease, Front. Cell. Neurosci., 15, 702824, https://doi.org/10.3389/fncel.2021.702824.
Babaei, P. (2021) NMDA and AMPA receptors dysregulation in Alzheimer’s disease, Eur. J. Pharmacol., 908, 174310, https://doi.org/10.1016/j.ejphar.2021.174310.
Kumar, A. (2015) NMDA receptor function during senescence: implication on cognitive performance, Front. Neurosci., 9, 473, https://doi.org/10.3389/fnins.2015.00473.
Kumar, A., and Foster, T. C. (2018) Alteration in NMDA receptor mediated glutamatergic neurotransmission in the hippocampus during senescence, Neurochem. Res., 44, 38-48, https://doi.org/10.1007/S11064-018-2634-4.
Avila, J., Llorens-Martín, M., Pallas-Bazarra, N., Bolos, M., Perea, J. R., Rodríguez-Matellan, A., and Hernandez, F. (2017) Cognitive decline in neuronal aging and Alzheimer’s disease: role of NMDA receptors and associated proteins, Front. Neurosci., 11, 626, https://doi.org/10.3389/fnins.2017.00626.
Cercato, M. C., Vázquez, C. A., Kornisiuk, E., Aguirre, A. I., Colettis, N., Snitcofsky, M., Jerusalinsky, D. A., and Baez, M. V. (2017) GluN1 and GluN2A NMDA receptor subunits increase in the hippocampus during memory consolidation in the rat, Front. Behav. Neurosci., 10, 242, https://doi.org/10.3389/fnbeh.2016.00242.
Ge, Y., and Wang, Y. T. (2023) GluN2B-containing NMDARs in the mammalian brain: pharmacology, physiology, and pathology, Front. Mol. Neurosci., 16, 1190324, https://doi.org/10.3389/fnmol.2023.1190324.
Yeung, J. H. Y., Walby, J. L., Palpagama, T. H., Turner, C., Waldvogel, H. J., Faull, R. L. M., and Kwakowsky, A. (2021) Glutamatergic receptor expression changes in the Alzheimer’s disease hippocampus and entorhinal cortex, Brain Pathol., 31, e13005, https://doi.org/10.1111/BPA.13005.
Qu, W., Yuan, B., Liu, J., Liu, Q., Zhang, X., Cui, R., Yang, W., and Li, B. (2021) Emerging role of AMPA receptor subunit GluA1 in synaptic plasticity: implications for Alzheimer’s disease, Cell Prolif., 54, e12959, https://doi.org/10.1111/cpr.12959.
Li, Y., Sun, H., Chen, Z., Xu, H., Bu, G., and Zheng, H. (2016) Implications of GABAergic neurotransmission in Alzheimer’s disease, Front. Aging Neurosci., 8, 31, https://doi.org/10.3389/fnagi.2016.00031.
Bi, D., Wen, L., Wu, Z., and Shen, Y. (2020) GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease, Alzheimers Dement., 16, 1312-1329, https://doi.org/10.1002/alz.12088.
Lee, S. E., Lee, Y., and Lee, G. H. (2019) The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain, Arch. Pharm. Res., 42, 1031-1039, https://doi.org/10.1007/s12272-019-01196-z.
Chattopadhyaya, B., Di Cristo, G., Wu, C. Z., Knott, G., Kuhlman, S., Fu, Y., Palmiter, R. D., and Huang, Z. J. (2007) GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex, Neuron, 54, 889-903, https://doi.org/10.1016/j.neuron.2007.05.015.
Lau, C. G., and Murthy, V. N. (2012) Activity-dependent regulation of inhibition via GAD67, J. Neurosci., 32, 8521-8531, https://doi.org/10.1523/JNEUROSCI.1245-12.2012.
Sandhu, K. V., Lang, D., Müller, B., Nullmeier, S., Yanagawa, Y., Schwegler, H., and Stork, O. (2014) Glutamic acid decarboxylase 67 haplodeficiency impairs social behavior in mice, Genes Brain Behav., 13, 439-450, https://doi.org/10.1111/GBB.12131.
Kash, S. F., Johnson, R. S., Tecott, L. H., Noebels, J. L., Mayfield, R. D., Hanahan, D., and Baekkeskov, S. (1997) Epilepsy in mice deficient in the 65-KDa isoform of glutamic acid decarboxylase, Proc. Natl. Acad. Sci. USA, 94, 14060-14065, https://doi.org/10.1073/PNAS.94.25.14060.
Toritsuka, M., Yoshino, H., Makinodan, M., Ikawa, D., Kimoto, S., Yamamuro, K., Okamura, K., Akamatsu, W., Okada, Y., Matsumoto, T., Hashimoto, K., Ogawa, Y., Saito, Y., Watanabe, K., Aoki, C., Takada, R., Fukami, S. I., Hamano-Iwasa, K., Okano, H., and Kishimoto, T. (2021) Developmental dysregulation of excitatory-to-inhibitory GABA-polarity switch may underlie schizophrenia pathology: a monozygotic-twin discordant case analysis in human IPS cell-derived neurons, Neurochem. Int., 150, e105179, https://doi.org/10.1016/J.NEUINT.2021.105179.
Benes, F. M., Lim, B., Matzilevich, D., Walsh, J. P., Subburaju, S., and Minns, M. (2007) Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars, Proc. Natl. Acad. Sci. USA, 104, 10164-10169, https://doi.org/10.1073/pnas.0703806104.
Lanoue, A. C., Dumitriu, A., Myers, R. H., Soghomonian, J. J. (2010) Decreased glutamic acid decarboxylase MRNA expression in prefrontal cortex in Parkinson’s disease, Exp. Neurol., 226, 207-217, https://doi.org/10.1016/j.expneurol.2010.09.001.
Wang, Y., Wu, Z., Bai, Y. T., Wu, G. Y., and Chen, G. (2017) Gad67 haploinsufficiency reduces amyloid pathology and rescues olfactory memory deficits in a mouse model of Alzheimer’s disease, Mol. Neurodegener., 12, 73, https://doi.org/10.1186/s13024-017-0213-9.
Ethiraj, J., Palpagama, T. H., Turner, C., van der Werf, B., Waldvogel, H. J., Faull, R. L. M., and Kwakowsky, A. (2021) The effect of age and sex on the expression of GABA signaling components in the human hippocampus and entorhinal cortex, Sci. Rep., 11, 21470, https://doi.org/10.1038/s41598-021-00792-8.
Krantic, S., Isorce, N., Mechawar, N., Davoli, M. A., Vignault, E., Albuquerque, M., Chabot, J. G., Moyse, E., Chauvin, J. P., Aubert, I., McLaurin, J., and Quirion, R. (2012) Hippocampal GABAergic neurons are susceptible to amyloid-β Toxicity in vitro and are decreased in number in the Alzheimer’s disease TgCRND8 mouse model, J. Alzheimers Dis., 29, 293-308, https://doi.org/10.3233/JAD-2011-110830.
Ulrich, D. (2015) Amyloid-β impairs synaptic inhibition via GABAA receptor endocytosis, J. Neurosci., 35, 9205-9210, https://doi.org/10.1523/JNEUROSCI.0950-15.2015.
Palpagama, T. H., Sagniez, M., Kim, S., Waldvogel, H. J., Faull, R. L., and Kwakowsky, A. (2019) GABAA receptors are well preserved in the hippocampus of aged mice, eNeuro, 6, 1-13, https://doi.org/10.1523/ENEURO.0496-18.2019.
Rissman, R. A., and Mobley, W. C. (2011) Implications for treatment: GABAA receptors in aging, down syndrome and Alzheimer’s disease, J. Neurochem., 117, 613-622, https://doi.org/10.1111/J.1471-4159.2011.07237.X.
Stefanova, N. A., Kozhevnikova, O. S., Vitovtov, A. O., Maksimova, K. Y., Logvinov, S. V., Rudnitskaya, E. A., Korbolina, E. E., Muraleva, N. A., and Kolosova, N. G. (2014) Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease, Cell Cycle, 13, 898-909, https://doi.org/10.4161/CC.28255.
Neff, R. A., Wang, M., Vatansever, S., Guo, L., Ming, C., Wang, Q., Wang, E., Horgusluoglu-Moloch, E., Song, W. M., Li, A., Castranio, E. L., Tcw, J., Ho, L., Goate, A., Fossati, V., Noggle, S., Gandy, S., Ehrlich, M. E., Katsel, P., Schadt, E., Cai, D., Brennand, K. J., Haroutunian, V., and Zhang, B. (2021) Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets, Sci. Adv., 7, eabb5398, https://doi.org/10.1126/sciadv.abb5398.
Funding
The work was financially supported by the Russian Science Foundation (project no. 19-15-00044).
Author information
Authors and Affiliations
Contributions
A.O.B. and N.A.S. experimental work; A.O.B., N.A.S., D.V.T., and N.G.K. discussion of research results; A.O.B. and D.V.T. writing the manuscript; N.G.K., N.A.S., and D.V.T. editing the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All studies were carried out with OXYS and Wistar (control) male rats on the basis of the Center for Collective Use “Gene Pools of Laboratory Animals”, Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, in accordance with the ethical standards of European Union Directive 2010/63/EU of September 22, 2010. All experiments were approved and performed in accordance with the guidelines of the Ethics Committee on Animal Trials at the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia (Resolution no. 12000-496 of April 2, 1980; Presidium of the Russian Academy of Sciences).
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burnyasheva, A.O., Stefanova, N.A., Kolosova, N.G. et al. Changes in the Glutamate/GABA System in the Hippocampus of Rats with Age and during Alzheimer’s Disease Signs Development. Biochemistry Moscow 88, 1972–1986 (2023). https://doi.org/10.1134/S0006297923120027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923120027