Abstract
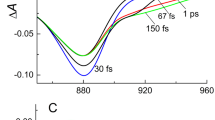
Process of photosynthesis in the green bacteria Chloroflexus (Cfx.) aurantiacus starts from absorption of light by chlorosomes, peripheral antennas consisting of thousands of bacteriochlorophyll c (BChl c) molecules combined into oligomeric structures. In this case, the excited states are formed in BChl c, energy of which migrates along the chlorosome towards the baseplate and further to the reaction center, where the primary charge separation occurs. Energy migration is accompanied by non-radiative electronic transitions between the numerous exciton states, that is, exciton relaxation. In this work, we studied dynamics of the exciton relaxation in Cfx. aurantiacus chlorosomes using differential femtosecond spectroscopy at cryogenic temperature (80 K). Chlorosomes were excited by 20-fs light pulses at wavelengths in the range from 660 to 750 nm, and differential (light-dark) absorption kinetics were measured at a wavelength of 755 nm. Mathematical analysis of the obtained data revealed kinetic components with characteristic times of 140, 220, and 320 fs, which are responsible for exciton relaxation. As the excitation wavelength decreased, the number and relative contribution of these components increased. Theoretical modelling of the obtained data was carried out based of the cylindrical model of BChl c. Nonradiative transitions between the groups of exciton bands were described by a system of kinetic equations. The model that takes into account energy and structural disorder of chlorosomes turned out to be the most adequate.







Similar content being viewed by others
Abbreviations
- BChl:
-
bacteriochlorophyll
- ΔA :
-
absorption difference (light-dark)
- τ1-6 :
-
characteristic time of the kinetic components
- λex :
-
excitation wavelength
- λprobe :
-
probe wavelength
References
Yakovlev, A. G., Taisova, A. S., and Fetisova, Z. G. (2020) Energy transfer in light-harvesting apparats of natural photosynthesis [in Russian], Usp. Sovr. Biol., 140, 166-182, https://doi.org/10.31857/S0042132420020088.
Frigaard, N.-U., and Bryant, D. (2006) Chlorosomes: antenna organelles in green photosynthetic bacteria, in Complex Intracellular Structures in Prokaryotes. Microbiology Monographs (Shively, J. M., ed) Springer, Berlin, pp. 79-114, https://doi.org/10.1007/7171_021.
Blankenship, R. E., Olson, J. M., and Miller, M. (1995) Antenna complexes from green photosynthetic bacteria, in Anoxygenic Photosynthetic Bacteria (Blankenship, R. E., Madigan, M. T., Bauer, C. E., eds) Kluwer Academic Publishers, Dordrecht, pp. 399-435, https://doi.org/10.1007/0-306-47954-0_20.
Fetisova, Z., and Mauring, K. (1992) Experimental evidence of oligomeric organization of antenna bacteriochlorophyll c in green bacterium Chloroflexus aurantiacus by spectral hole burning, FEBS Lett., 307, 371-374, https://doi.org/10.1016/0014-5793(92)80715-S.
Fetisova, Z., Freiberg, A., and Timpmann, K. (1988) Long-range molecular order as an efficient strategy for light harvesting in photosynthesis, Nature (London), 334, 633-634, https://doi.org/10.1038/334633a0.
Van Dorssen, R. J., Vasmel, H., and Amesz, J. (1986) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus. II. The chlorosome, Photosynth. Res., 9, 33-45, https://doi.org/10.1007/BF00029729.
Krasnovsky, A., and Bystrova, M. (1980) Self-assembly of chlorophyll aggregated structures, BioSystems, 12, 181-194, https://doi.org/10.1016/0303-2647(80)90016-7.
Smith, K., Kehres, L., and Fajer, J. (1983) Aggregation of bacteriochlorophylls c, d or e. Models for the antenna chlorophylls of green and brown photosynthetic bacteria, J. Am. Chem. Soc., 105, 1387-1389, https://doi.org/10.1021/ja00343a062.
Olson, J. M. (1998) Chlorophyll organization and function in green photosynthetic bacteria, Photochem. Photobiol., 67, 61-75, https://doi.org/10.1111/j.1751-1097.1998.tb05166.x.
Pierson, B., and Castenholz, R. (I974) Pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium, Arch. Microbiol., 100, 283-305, https://doi.org/10.1007/BF00446324.
Oelze, J. (1992) Light and oxygen regulation of the synthesis of bacteriochlorophyll a and bacteriochlorophyll c in Chloroflexus aurantiacus, J. Bacteriol., 174, 5021-5026, https://doi.org/10.1128/jb.174.15.5021-5026.1992.
Fetisova, Z. G., Freiberg, A. M., Mauring, K., Novoderezhkin, V. I., Taisova, A. S., and Timpmann, K. E. (1996) Excitation energy transfer in chlorosomes of green bacteria: Theoretical and experimental studies, Biophys. J., 71, 995-1010, https://doi.org/10.1016/S0006-3495(96)79301-3.
Sprague, S., Staehelin, L., DiBartolomeis, M., and Fuller, R. (1981) Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus, J. Bacteriol., 147, 1021-1031, https://doi.org/10.1128/jb.147.3.1021-1031.1981.
Staehelin, L., Golecki, J., Fuller, R., and Drews, G. (1978) Visualization of the supramolecular architecture of chlorosomes (Chlorobium type vesicles) in freeze-fractured cells of Chloroflexus aurantiacus, Arch. Microbiol., 119, 269-277, https://doi.org/10.1007/BF00405406.
Olson, J. M. (1980) Chlorophyll organization in green photosynthetic bacteria, Biochim. Biophys. Acta, 594, 33-51, https://doi.org/10.1016/0304-4173(80)90012-9.
Psencik, J., Ikonen, T. P., Laurinmaki, P., Merckel, M. C., Butcher, S. J., Serimaa, R. E., and Tuma, R. (2004) Lamellar organization of pigments in chlorosomes, the light harvesting complexes of green photosynthetic bacteria, Biophys. J., 87, 1165-1172, https://doi.org/10.1529/biophysj.104.040956.
Günther, L., Jendrny, M., Bloemsma, E., Tank, M., Oostergetel, G., Bryant, D., Knoester, J., and Köhler, J. (2016) Structure of light-harvesting aggregates in individual chlorosomes, J. Phys. Chem. B, 120, 5367-5376, https://doi.org/10.1021/acs.jpcb.6b03718.
Sawaya, N., Huh, J., Fujita, T., Saikin, S., and Aspuru-Guzik, A. (2015) Fast delocalization leads to robust long-range excitonic transfer in a large quantum chlorosome model, Nano Lett., 15, 1722-1729, https://doi.org/10.1021/nl504399d.
Fujita, T., Huh, J., Saikin, S., Brookes, J., and Aspuru-Guzik, A. (2014) Theoretical characterization of excitation energy transfer in chlorosome light-harvesting antennae from green sulfur bacteria, Photosynth. Res., 120, 273-289, https://doi.org/10.1007/s11120-014-9978-7.
Prokhorenko, V. I., Steensgaard, D. B., and Holzwarth, A. R. (2000) Exciton dynamics in the chlorosomal antennae of the green bacteria Chloroflexus aurantiacus and Chlorobium tepidum, Biophys. J., 79, 2105-2120, https://doi.org/10.1016/S0006-3495(00)76458-7.
Savikhin, S., Zhu, Y., Lin, S., Blankenship, R. E., and Struve, W. S. (1994) Femtosecond spectroscopy of chlorosome antennas from the green photosynthetic bacterium Chloroflexus aurantiacus, J. Phys. Chem., 98, 10322-10334, https://doi.org/10.1021/j100091a056.
Savikhin, S., Zhu, Y., Blankenship, R. E., and Struve, W. S. (1996) Intraband energy transfers in the BChl c antenna of chlorosomes from the green photosynthetic bacterium Chloroflexus aurantiacus, J. Phys. Chem., 100, 17978-17980, https://doi.org/10.1021/jp961752b.
Savikhin, S., Buck, D. R., Struve, W. S., Blankenship, R. E., Taisova, A. S., Novoderezhkin, V. I., and Fetisova, Z. G. (1998) Exciton delocalization in the bacteriochlorophyll c antenna of the green bacterium Chloroflexus aurantiacus as revealed by ultrafast pump-probe spectroscopy, FEBS Lett., 430, 323-326, https://doi.org/10.1016/S0014-5793(98)00691-7.
Yakovlev, A., Taisova, A., and Fetisova, Z. (2002) Light control over the size of an antenna unit building block as an effecient strategy for light harvesting in photosynthesis, FEBS Lett., 512, 129-132, https://doi.org/10.1016/S0014-5793(02)02238-X.
Yakovlev, A., Novoderezhkin, V., Taisova, A., and Fetisova, Z. (2002) Exciton dynamics in the chlorosomal antenna of the green bacterium Chloroflexus aurantiacus: experimental and theoretical studies of femtosecond pump-probe spectra, Photosynth. Res., 71, 19-32, https://doi.org/10.1023/A:1014995328869.
Psencik, J., Ma, Y. Z., Arellano, J. B., Garcia-Gil, J., Holzwarth, A. R., and Gillbro, T. (2002) Excitation energy transfer in chlorosomes of Chlorobium phaeobacteroides strain CL1401: the role of carotenoids, Photosynth. Res., 71, 5-18, https://doi.org/10.1023/A:1014943312031.
Psencik, J., Ma, Y. Z., Arellano, J. B., Hala, J., and Gillbro, T. (2003) Excitation energy transfer dynamics and excited-state structure in chlorosomes of Chlorobium phaeobacteroides, Biophys. J., 84, 1161-1179, https://doi.org/10.1016/S0006-3495(03)74931-5.
Martiskainen, J., Linnanto, J., Kananavičius, R., Lehtovuori, V., and Korppi-Tommola, J. (2009) Excitation energy transfer in isolated chlorosomes from Chloroflexus aurantiacus, Chem. Phys. Lett., 477, 216-220, https://doi.org/10.1016/j.cplett.2009.06.080.
Martiskainen, J., Linnanto, J., Aumanen, V., Myllyperkiö, P., and Korppi-Tommola, J. (2012) Excitation energy transfer in isolated chlorosomes from Chlorobaculum tepidum and Prosthecochloris aestuarii, Photochem. Photobiol., 88, 675-683, https://doi.org/10.1111/j.1751-1097.2012.01098.x.
Linnanto, J. V., and Korppi-Tommola, J. E. I. (2012) Exciton description of excitation energy transfer in the photosynthetic units of green sulfur bacteria and filamentous anoxygenic phototrophs, J. Phys. Chem. B, 117, 11144-11161, https://doi.org/10.1021/jp4011394.
Yakovlev, A. G., Taisova, A. S., Shuvalov, V. A., and Fetisova, Z. G. (2019) Ultrafast excited-state dynamics in chlorosomes isolated from the photosynthetic filamentous green bacterium Chloroflexus aurantiacus, Physiologia Plantarum, 166, 12-21, https://doi.org/10.1111/ppl.12887.
Yakovlev, A. G., Taisova, A. S., and Fetisova, Z. G. (2021) Utilization of blue-green light by chlorosomes from the photosynthetic bacterium Chloroflexus aurantiacus: Ultrafast excitation energy conversion and transfer, Biochim. Biophys. Acta Bioenergetics, 1862, 148396, https://doi.org/10.1016/j.bbabio.2021.148396.
Causgrove, T. P., Brune, D. C., Wang, J., Wittmershaus, B. P., and Blankenship, R. E. (1990) Energy transfer kinetics in whole cells and isolated chlorosomes of green photosynthetic bacteria, Photosynth. Res., 26, 39-48, https://doi.org/10.1007/BF00048975.
Taisova, A. S., Keppen, O. I., Lukashev, E. P., Arutyunyan, A. M., and Fetisova, Z. G. (2002) Study of the chlorosomal antenna of the green mesophilic filamentous bacterium Oscillochloris trichoides, Photosynth. Res., 74, 73-85, https://doi.org/10.1023/A:1020805525800.
Mukamel, S. (1995) Principles of Nonlinear Optical Spectroscopy, Oxford University Press, New York/Oxford.
Mauring, K., Novoderezhkin, V., Taisova, A., and Fetisova, Z. (1999) Exciton levels structure of antenna bacteriochlorophyll c aggregates in the green bacterium Chloroflexus aurantiacus as probed by 1.8-293 K fluorescence spectroscopy, FEBS Lett., 456, 239-242, https://doi.org/10.1016/S0014-5793(99)00953-9.
May, V. (2014) Kinetic theory of exciton–exciton annihilation, J. Chem. Phys., 140, 054103, https://doi.org/10.1063/1.4863259.
Yakovlev, A., Taisova, A., Arutyunyan, A., Shuvalov, V., and Fetisova, Z. (2017) Variability of aggregation extent of light-harvesting pigments in peripheral antenna of Chloroflexus aurantiacus, Photosynth. Res., 133, 343-356, https://doi.org/10.1007/s11120-017-0374-y.
Jendrny, M., Aartsma, T. J., and Köhler, J. (2014) Insights into the excitonic states of individual chlorosomes from Chlorobaculum tepidum, Biophys. J., 106, 1921-1927, https://doi.org/10.1016/j.bpj.2014.03.020.
Petrov, E. G. (1984) Fisika Perenosa Zaryadov v Biosistemakh, Naukova Dumka, Kiev.
Struve, W. S. (1995) Vibrational equilibration in absorption difference spectra of chlorophyll a, Biophys. J., 69, 2739-2744, https://doi.org/10.1016/S0006-3495(95)80145-1.
Márquez, A.S., Chen, L., Sun, K., and Zhao, Y. (2016) Probing ultrafast excitation energy transfer of the chlorosome with exciton–phonon variational dynamics, Phys. Chem. Chem. Phys., 18, 20298, https://doi.org/10.1039/C5CP06491K.
Dostál, J., Mančal, T., Augulis, R., Vácha, F., Pšenčík, J., and Zigmantas, D. (2012) Two-dimensional electronic spectroscopy reveals ultrafast energy diffusion in chlorosomes, J. Am. Chem. Soc., 34, 11611-11617, https://doi.org/10.1021/ja3025627.
Dong, L.-Q., Niu, K., and Cong, S.-L. (2007) Theoretical analysis of internal conversion pathways and vibrational relaxation process of chlorophyll-a in ethyl ether solvent, Chem. Phys. Lett., 440, 150-154, https://doi.org/10.1016/j.cplett.2007.04.021.
Cherepy, N. J., Du, M., Holzwarth, A. R., and Mathies, R. A. (1996) Near-infrared resonance Raman spectra of chlorosomes: probing nuclear coupling in electronic energy transfer, J. Phys. Chem., 100, 4662-4671, https://doi.org/10.1021/jp952992e.
Gülen, D. (2006) Significance of the excitonic intensity borrowing in the J-/H-aggregates of bacteriochlorophylls/chlorophylls, Photosynth. Res., 87, 205-214, https://doi.org/10.1007/s11120-005-8408-2.
Yakovlev, A. G., Taisova, A. S., Shuvalov, V. A., and Fetisova, Z. G. (2018) Estimation of the bacteriochlorophyll c oligomerisation extent in Chloroflexus aurantiacus chlorosomes by very low-frequency vibrations of the pigment molecules: A new approach, Biophys. Chem., 240, 1-8, https://doi.org/10.1016/j.bpc.2018.05.004.
Sokolov, A. A., Ternovm I. M. (1970) Kvantovaya Mekhanica i Atomnaya Fisika [in Russian], Prosveschenie, Moscow.
Acknowledgments
The authors express their deep gratitude to the late Academician V.A. Shuvalov for general support and constant attention to their work.
Funding
The study was financially supported by the State Budget Project No. AAAA-A17-117120540070-0 (“Photobiophysics of solar energy conversion in living systems”).
Author information
Authors and Affiliations
Contributions
Z.G.F. – supervision of the work, discussion of the results; A.G.Y. – supervision, conducting experiments and calculations, discussion of the results, writing an article; A.S.T. – cultivation of bacteria, preparing samples, writing the article.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. This article does not contain description of studies involving humans or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yakovlev, A.G., Taisova, A.S. & Fetisova, Z.G. Femtosecond Exciton Relaxation in Chlorosomes of the Photosynthetic Green Bacterium Chloroflexus aurantiacus. Biochemistry Moscow 88, 704–715 (2023). https://doi.org/10.1134/S0006297923050139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923050139