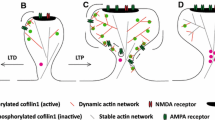
Abstract
Modulation of presynaptic short-term plasticity induced by actin polymerization was studied in rat hippocampal slices using the paired-pulse paradigm. Schaffer collaterals were stimulated with paired pulses with a 70-ms interstimulus interval every 30 s before and during perfusion with jasplakinolide, an activator of actin polymerization. Jasplakinolide application resulted in the increase in the amplitudes of CA3-CA1 responses (potentiation) accompanied by a decrease in the paired-pulse facilitation, suggesting induction of presynaptic modifications. Jasplakinolide-induced potentiation depended on the initial paired-pulse rate. These data indicate that the jasplakinolide-mediated changes in actin polymerization increased the probability of neurotransmitter release. Less typical for CA3-CA1 synapses responses, such as a very low paired-pulse ratio (close to 1 or even lower) or even paired-pulse depression, were affected differently. Thus, jasplakinolide caused potentiation of the second, but not the first response to the paired stimulus, which increased the paired-pulse ratio from 0.8 to 1.0 on average, suggesting a negative impact of jasplakinolide on the mechanisms promoting paired-pulse depression. In general, actin polymerization facilitated potentiation, although the patterns of potentiation differed depending on the initial synapse characteristics. We conclude that in addition to the increase in the neurotransmitter release probability, jasplakinolide induced other actin polymerization-dependent mechanisms, including those involved in the paired-pulse depression.



Similar content being viewed by others
Abbreviations
- LTP:
-
long-term potentiation
- PPD:
-
paired-pulse depression
- PPF:
-
paired-pulse facilitation
- PPR:
-
paired-pulse ratio
References
Choquet, D., and Triller, A. (2013) The dynamic synapse, Neuron, 80, 691-703, https://doi.org/10.1016/j.neuron.2013.10.013.
Meyer, D., Bonhoeffer, T., and Scheuss, V. (2014) Balance and stability of synaptic structures during synaptic plasticity, Neuron, 82, 430-443, https://doi.org/10.1016/j.neuron.2014.02.031.
Kudryashova, I. V. (2019) The molecular basis of destabilization of synapses as a factor of structural plasticity, Neurochem. J., 13, 3-13, https://doi.org/10.1134/S1819712419010136.
Dillon, C., and Goda, Y. (2005) The actin cytoskeleton: integrating form and function at the synapse, Annu. Rev. Neurosci., 28, 25-55, https://doi.org/10.1146/annurev.neuro.28.061604.135757.
Cingolani, L. A., and Goda, Y. (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy, Nat. Rev. Neurosci., 9, 344-356, https://doi.org/10.1038/nrn2373.
Ramachandran, B., and Frey, J. U. (2009) Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro, J. Neurosci., 29, 12167-12173, https://doi.org/10.1523/JNEUROSCI.2045-09.2009.
Rex, C. S., Gavin, C. F., Rubio, M. D., Kramar, E. A., Chen, L. Y., et al. (2010) Myosin IIB regulates actin dynamics during synaptic plasticity and memory formation, Neuron, 67, 603-617, https://doi.org/10.1016/j.neuron.2010.07.016.
Krucker, T., Siggins, G. R., and Halpain, S. (2000) Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus, Proc. Natl. Acad. Sci. USA, 97, 6856-6861, https://doi.org/10.1073/pnas.100139797.
Kramar, E. A., Lin, B., Rex, C. S., Gall, C. M., and Lynch, G. (2006) Integrin-driven actin polymerization consolidates long-term potentiation, Proc. Natl. Acad. Sci. USA, 103, 5579-5584, https://doi.org/10.1073/pnas.0601354103.
Messaoudi, E., Kanhema, T., Soule, J., Tiron, A., Dagyte, G., et al. (2007) Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo, J. Neurosci., 27, 10445-10455, https://doi.org/10.1523/JNEUROSCI.2883-07.2007.
Galvez, B., Gross, N., and Sumikawa, K. (2016) Activation of α7 nicotinic acetylcholine receptors protects potentiated synapses from depotentiation during theta pattern stimulation in the hippocampal CA1 region of rats, Neuropharmacology, 105, 378-387, https://doi.org/10.1016/j.neuropharm.2016.02.008.
Shupliakov, O., Bloom, O., Gustafsson, J. S., Kjaerulff, O., Low, P., et al. (2002) Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton, Proc. Natl. Acad. Sci. USA, 99, 14476-14481, https://doi.org/10.1073/pnas.212381799.
Böhme, M. A., McCarthy, A. W., Grasskamp, A. T., Beuschel, C. B., Goel, P., et al. (2019) Rapid active zone remodeling consolidates presynaptic potentiation, Nat. Commun., 10, 1085, https://doi.org/10.1038/s41467-019-08977-6.
Morales, M., Colicos, M. A., and Goda, Y. (2000) Actin-dependent regulation of neurotransmitter release at central synapses, Neuron, 27, 539-550, https://doi.org/10.1016/S0896-6273(00)00064-7.
Sankaranarayanan, S., Atluri, P. P., and Ryan, T. A. (2003) Actin has a molecular scaffolding, not propulsive, role in presynaptic function, Nat. Neurosci., 6, 127-135, https://doi.org/10.1038/nn1002.
Rust, M. B., and Maritzen, T. (2015) Relevance of presynaptic actin dynamics for synapse function and mouse behavior, Exp. Cell Res., 335, 165-171, https://doi.org/10.1016/j.yexcr.2014.12.020.
Malinow, R., and Tsien, R. W. (1990) Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices, Nature, 46, 177-180, https://doi.org/10.1038/346177a0.
Malgaroli, A., Ting, A. E., Wendland, B., Bergamaschi, A., Villa, A., et al. (1995) Presynaptic component of long-term potentiation visualized at individual hippocampal synapses, Science, 268, 1624-1628, https://doi.org/10.1126/science.7777862.
Nicoll, R. A., and Malenka, R. C. (1995) Contrasting properties of two forms of long-term potentiation in the hippocampus, Nature, 377, 115-118, https://doi.org/10.1038/377115a0.
Blundon, J. A., and Zakharenko, S. S. (2008) Dissecting the components of long-term potentiation, Neuroscientist, 14, 598-608, https://doi.org/10.1177/1073858408320643.
Sokolov, M. V., Rossokhin, A. V., Astrelin, A. V., Frey, J. U., and Voronin, L. L. (2002) Quantal analysis suggests strong involvement of presynaptic mechanisms during the initial maintenance of long-term potentiation in rat hippocampal CA1 area in vitro, Brain Res., 957, 61-75, https://doi.org/10.1016/S0006-8993(02)03600-4.
Emptage, N. J., Reid, C. A., Fine, A., and Bliss, T. V. (2003) Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer-associational synapses, Neuron, 38, 797-804, https://doi.org/10.1016/S0896-6273(03)00325-8.
Stanton, P. K., Winterer, J., Zhang, X. L., and Muller, W. (2005) Imaging LTP of presynaptic release of FM1-43 from the rapidly recycling vesicle pool of Schaffer collateral-CA1 synapses in rat hippocampal slices, Eur. J. Neurosci., 22, 2451-2461, https://doi.org/10.1111/j.1460-9568.2005.04437.x.
Bayazitov, I. T., Richardson, R. J., Fricke, R. G., and Zakharenko, S. S. (2007) Slow presynaptic and fast postsynaptic components of compound long-term potentiation, J. Neurosci., 27, 11510-11521, https://doi.org/10.1523/JNEUROSCI.3077-07.2007.
Kudryashova, I. V., Onufriev, M. V., and Gulyaeva, N. V. (2014) Caspase-3 and calpain: differently directed involvement in presynaptic long-term plasticity, Neurochem. J., 8, 162-167, https://doi.org/10.1134/S181971241403009X.
Kuhnt, U., and Voronin, L. L. (1994) Interaction between paired-pulse facilitation and long-term potentiation in area CA1 of guinea-pig hippocampal slices: application of quantal analysis, Neuroscience, 62, 391-397, https://doi.org/10.1016/0306-4522(94)90374-3.
Schulz, P. E., Cook, E. P., and Johnston, D. (1994) Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation, J. Neurosci., 14, 5325-5337, https://doi.org/10.1523/JNEUROSCI.14-09-05325.1994.
Yao, J., Qi, J., and Chen, G. (2006) Actin-Dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons, J. Neurosci., 26, 8137-8147, https://doi.org/10.1523/JNEUROSCI.1183-06.2006.
Guzman, G. A., Guzman, R. E., Jordan, N., and Hidalgo, P. A. (2019) Tripartite interaction among the calcium channel α1- and β-subunits and F-Actin increases the readily releasable pool of vesicles and its recovery after depletion, Front. Cell Neurosci., 13, 125, https://doi.org/10.3389/fncel.2019.00125.
Zucker, R. S., and Regehr, W. G. (2002) Short-term synaptic plasticity, Annu. Rev. Physiol., 64, 355-405, https://doi.org/10.1146/annurev.physiol.64.092501.114547.
Fioravante, D., and Regehr, W. G. (2011) Short-term forms of presynaptic plasticity, Curr. Opin. Neurobiol., 21, 269-274, https://doi.org/10.1016/j.conb.2011.02.003.
Bubb, M. R., Spector, I., Beyer, B. B., and Fosen, K. M. (2000) Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations, J. Biol. Chem., 275, 5163-5170, https://doi.org/10.1074/jbc.275.7.5163.
Lazarevic, V., Pothula, S., Andres-Alonso, M., and Fejtova, A. (2013) Molecular mechanisms driving homeostatic plasticity of neurotransmitter release, Front. Cell Neurosci., 7, 244, https://doi.org/10.3389/fncel.2013.00244.
Davis, G. W., and Muller, M. (2015) Homeostatic control of presynaptic neurotransmitter release, Annu. Rev. Physiol., 77, 251-270, https://doi.org/10.1146/annurev-physiol-021014-071740.
Ortega, J. M., Genç, Ö., and Davis, G. W. (2018) Molecular mechanisms that stabilize short term synaptic plasticity during presynaptic homeostatic plasticity, eLife, 7, e40385, https://doi.org/10.7554/eLife.40385.
Goel, P., Bergeron, D. D., Böhme, M. A., Nunnelly, L., Lehmann, M., et al. (2019) Homeostatic scaling of active zone scaffolds maintains global synaptic strength, J. Cell Biol., 18, 1706-1724, https://doi.org/10.1083/jcb.201807165.
Ivanov, A., Esclapez, M., Pellegrino, Ch., Shirao, T., and Ferhat, L. (2009) Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons, J. Cell Sci., 122, 524-534, https://doi.org/10.1242/jcs.033464.
Bleckert, A., Photowala, H., and Alford, S. (2012) Dual pools of actin at presynaptic terminals, J. Neurophysiol., 107, 3479-3492, https://doi.org/10.1152/jn.00789.2011.
Andrade, A. L., and Rossi, D. J. (2010) Simulated ischaemia induces Ca2+-independent glutamatergic vesicle release through actin filament depolymerization in area CA1 of the hippocampus, J. Physiol., 588, 1499-1514, https://doi.org/10.1113/jphysiol.2010.187609.
Rex, C. S., Chen, L. Y., Sharma, A., Liu, J., Babayan, A. H., et al. (2009) Different Rho GTPase–dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation, J. Cell Biol., 186, 85-97, https://doi.org/10.1083/jcb.200901084.
Gaviño, M. A., Ford, K. J., Archila, S., and Davis, G. W. (2015) Homeostatic synaptic depression is achieved through a regulated decrease in presynaptic calcium channel abundance, eLife, 4, e05473, https://doi.org/10.7554/eLife.05473.
Bloom, O., Evergren, E., Tomilin, N., Kjaerulff, O., Low, P., et al. (2003) Colocalization of synapsin and actin during synaptic vesicle recycling, J. Cell Biol., 161, 737-747, https://doi.org/10.1083/jcb.200212140.
Zhang, W., and Benson, D. L. (2001) Stages of synapse development defined by dependence on F-actin, J. Neurosci., 21, 5169-5181, https://doi.org/10.1523/JNEUROSCI.21-14-05169.2001.
Ackermann, F., Waites, C. L., and Garner, C. C. (2015) Presynaptic active zones in invertebrates and vertebrates, EMBO Rep., 16, 923-938, https://doi.org/10.15252/embr.201540434.
Südhof, T. C. (2012) The presynaptic active zone, Neuron, 75, 11-25, https://doi.org/10.1016/j.neuron.2012.06.012.
Lee, J. S., Ho, W. K., Neher, E., and Lee, S. H. (2013) Superpriming of synaptic vesicles after their recruitment to the readily releasable pool, Proc. Natl. Acad. Sci. USA, 110, 15079-15084, https://doi.org/10.1073/pnas.1314427110.
Ghelani, T., and Sigrist, S. J. (2018) Coupling the structural and functional assembly of synaptic release sites, Front. Neuroanat., 12, 81, https://doi.org/10.3389/fnana.2018.00081.
Halpain, S. (2003) Actin in a supporting role, Nat. Neurosci., 6, 101-102, https://doi.org/10.1038/nn0203-101.
Miki, T., Malagon, G., Pulido, C., Llano, I., Neher, E., et al. (2016) Actin- and myosin- dependent vesicle loading of presynaptic docking sites prior to exocytosis, Neuron, 91, 808-823, https://doi.org/10.1016/j.neuron.2016.07.033.
Holderith, N., Lorincz, A., Katona, G., Rózsa, B., Kulik, A., et al. (2012) Release probability of hippocampal glutamatergic terminals scales with the size of the active zone, Nat. Neurosci., 15, 988-997, https://doi.org/10.1038/nn.3137.
Körber, Ch., and Kuner, Th. (2016) Molecular machines regulating the release probability of synaptic vesicles at the active zone, Front. Synapt. Neurosci., 8, 5, https://doi.org/10.3389/fnsyn.2016.00005.
Bruckner, J. J., Zhan, H., Gratz, S. J., Rao, M., Ukken, F., et al. (2017) Fife organizes synaptic vesicles and calcium channels for high-probability neurotransmitter release, J. Cell Biol., 216, 231-246, https://doi.org/10.1083/jcb.201601098.
Gustafsson, B., Ma, R., and Hanse, E. (2019) The small and dynamic pre-primed pool at the release site; A useful concept to understand release probability and short-term synaptic plasticity?, Front. Synapt. Neurosci., 11, 7, https://doi.org/10.3389/fnsyn.2019.00007.
Mochida, S. (2019) Presynaptic calcium channels, Int. J. Mol. Sci., 20, 2217, https://doi.org/10.3390/ijms20092217.
Leal-Ortiz, S., Waites, C. L., Terry-Lorenzo, R., Zamorano, P., Gundelfinger, E. D., et al. (2008) Piccolo modulation of synapsin1a dynamics regulates synaptic vesicle exocytosis, J. Cell Biol., 181, 831-846, https://doi.org/10.1083/jcb.200711167.
Waites, C. L., Leal-Ortiz, S. A., Andlauer, T. F., Sigrist, S. J., and Garner, C. C. (2011) Piccolo regulates the dynamic assembly of presynaptic F-actin, J. Neurosci., 31, 14250-14263, https://doi.org/10.1523/JNEUROSCI.1835-11.2011.
Dason, J. S., Smith, A. J., Marin, L., and Charlton, M. P. (2014) Cholesterol and F-actin are required for clustering of recycling synaptic vesicle proteins in the presynaptic plasma membrane, J. Physiol., 592, 621-633, https://doi.org/10.1113/jphysiol.2013.265447.
Sakaba, T., and Neher, E. (2003) Involvement of actin polymerization in vesicle recruitment at the calyx of Held synapse, J. Neurosci., 23, 837-846, https://doi.org/10.1523/JNEUROSCI.23-03-00837.2003.
Gundelfinger, E. D., and Fejtova, A. (2012) Molecular organization and plasticity of the cytomatrix at the active zone, Curr. Opin. Neurobiol., 22, 423-430, https://doi.org/10.1016/j.conb.2011.10.005.
Lee, J. S., Ho, W. K., and Lee, S. H. (2012) Actin-dependent rapid recruitment of reluctant synaptic vesicles into a fast-releasing vesicle pool, Proc. Natl. Acad. Sci. USA, 109, E765-E774, https://doi.org/10.1073/pnas.1114072109.
Engholm-Keller, K., Waardenberg, A. J., Müller, J. A., Wark, J. R., Fernando, R. N., et al. (2019) The temporal profile of activity-dependent presynaptic phospho-signalling reveals long-lasting patterns of poststimulus regulation, PLoS Biol., 17, e3000170, https://doi.org/10.1371/journal.pbio.3000170.
Okamoto, K., Nagai, T., Miyawaki, A., and Hayashi, Y. (2004) Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity, Nat. Neurosci., 7, 1104-1112, https://doi.org/10.1038/nn1311.
Holzinger, A. (2009) Jasplakinolide: an actin-specific reagent that promotes actin polymerization, Meth. Mol. Biol., 586, 71-87, https://doi.org/10.1007/978-1-60761-376-3_4.
Kudryashova, I. V. (2021) The reorganization of the actin matrix as a factor of presynaptic plasticity, Neurochem. J., 15, 217-225, https://doi.org/10.1134/S1819712421030089.
Kudryashova, I. V. (2022) Inhibitory control of short-term plasticity during paired pulse stimulation depends on actin polymerization, Neurochem. J., 16, 136-146, https://doi.org/10.1134/S1819712422020106.
Sun, H. Y., Li, Q., Bartley, A. F., and Dobrunz, L. E. (2018) Target-cell-specific short-term plasticity reduces the excitatory drive onto CA1 interneurons relative to pyramidal cells during physiologically-derived spike trains, Neuroscience, 388, 430-447, https://doi.org/10.1016/j.neuroscience.2018.07.051.
Sullivan, J. M. (2007) A simple depletion model of the readily releasable pool of synaptic vesicles cannot account for paired-pulse depression, J. Neurophysiol., 97, 948-950, https://doi.org/10.1152/jn.00554.2006.
Catterall, W. A., Leal, K., and Nanou, E. (2013) Calcium channels and short-term synaptic plasticity, J. Biol. Chem., 288, 10742-10749, https://doi.org/10.1074/jbc.R112.411645.
Kaksonen, M., Toret, C. P., and Drubin, D. G. (2006) Harnessing actin dynamics for clathrin-mediated endocytosis, Nat. Rev. Mol. Cell Biol., 7, 404-414, https://doi.org/10.1038/nrm1940.
Verstegen, A. M., Tagliatti, E., Lignani, G., Marte, A., Stolero, T., et al. (2014) Phosphorylation of synapsin I by cyclin-dependent kinase-5 sets the ratio between the resting and recycling pools of synaptic vesicles at hippocampal synapses, J. Neurosci., 34, 7266-7280, https://doi.org/10.1523/JNEUROSCI.3973-13.2014.
Wu, X. S., Lee, S. H., Sheng, J., Zhang, Z., Zhao, W. D., et al. (2016) Actin is crucial for all kinetically distinguishable forms of endocytosis at synapses, Neuron, 92, 1020-1035, https://doi.org/10.1016/j.neuron.2016.10.014.
Kaeser, P. S., and Regehr, W. G. (2017) The readily releasable pool of synaptic vesicles, Curr. Opin. Neurobiol., 43, 63-70, https://doi.org/10.1016/j.conb.2016.12.012.
Bourne, J., Morgan, J. R., and Pieribone, V. A. (2006) Actin polymerization regulates clathrin coat maturation during early stages of synaptic vesicle recycling at lamprey synapses, J. Comp. Neurol., 497, 600-609, https://doi.org/10.1002/cne.21006.
Bahler, M., and Greengard, P. (1987) Synapsin I bundles F-actin in a phosphorylation- dependent manner, Nature, 326, 704-707, https://doi.org/10.1038/326704a0.
Rosahl, T. W., Geppert, M., Spillane, D., Herz, J., Hammer, R. E., et al. (1993) Short-term synaptic plasticity is altered in mice lacking synapsin I, Cell, 75, 661-670, https://doi.org/10.1016/0092-8674(93)90487-B.
Vasileva, M., Horstmann, H., Geumann, C., Gitler, D., and Kuner, T. (2012) Synapsin-dependent reserved pool of synaptic vesicles supports replenishment of the readily releasable pool under intense synaptic transmission, Eur. J. Neurosci., 36, 3005-3020, https://doi.org/10.1111/j.1460-9568.2012.08225.x.
Orlando, M., Lignani, G., Maragliano, L., Fassio, A., Onofri, F., et al. (2014) Functional role of ATP binding to synapsin I in synaptic vesicle trafficking and release dynamics, J. Neurosci., 34, 14752-14768, https://doi.org/10.1523/JNEUROSCI.1093-14.2014.
Watanabe, S., Rost, B. R., Camacho-Perez, M., Davis, M. W., Sohl-Kielczynski, B., et al. (2013) Ultrafast endocytosis at mouse hippocampal synapses, Nature, 504, 242-247, https://doi.org/10.1038/nature12809.
Rozov, A., and Burnashev, N. (1999) Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression, Nature, 401, 594-598, https://doi.org/10.1038/44151.
Brody, D. L., and Yue, D. T. (2000) Release-independent short-term synaptic depression in cultured hippocampal neurons, J. Neurosci., 20, 2480-2494, https://doi.org/10.1523/JNEUROSCI.20-07-02480.2000.
Nanou, E., and Catterall, W. A. (2018) Calcium channels, synaptic plasticity, and neuropsychiatric disease, Neuron, 98, 466-481, https://doi.org/10.1016/j.neuron.2018.03.017.
Chamberland, S., Evstratova, A., and Toth, K. (2014) Interplay between synchronization of multivesicular release and recruitment of additional release sites support short-term facilitation at hippocampal mossy fiber to CA3 pyramidal cells synapses, J. Neurosci., 34, 11032-11047, https://doi.org/10.1523/JNEUROSCI.0847-14.2014.
Rajappa, R., Gauthier-Kemper, A., Böning, D., Hüve, J., and Klingauf, J. (2016) Synaptophysin 1 clears synaptobrevin 2 from the presynaptic active zone to prevent short-term depression, Cell Rep., 14, 1369-1381, https://doi.org/10.1016/j.celrep.2016.01.031.
Hruska, M., Henderson, N., Le Marchand, S. J., Jafri, H., and Dalva, M. B. (2018) Synaptic nanomodules underlie the organization and plasticity of spine synapses, Nat. Neurosci., 21, 671-682, https://doi.org/10.1038/s41593-018-0138-9.
Catterall, W. A., and Few, A. P. (2008) Calcium channel regulation and presynaptic plasticity, Neuron, 59, 882-901, https://doi.org/10.1016/j.neuron.2008.09.005.
Eggermann, E., Bucurenciu, I., Goswami, S. P., and Jonas, P. (2012) Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses, Nat. Rev. Neurosci., 13, 7-21, https://doi.org/10.1038/nrn3125.
Scimemi, A., and Diamond, J. S. (2012) The number and organization of Ca2+ channels in the active zone shapes neurotransmitter release from schaffer collateral synapses, J. Neurosci., 32, 18157-18176, https://doi.org/10.1523/JNEUROSCI.3827-12.2012.
Yan, J., Leal, K., Magupalli, V. G., Nanou, E., Martinez, G. Q., et al. (2014) Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation, Mol. Cell Neurosci., 63, 124-131, https://doi.org/10.1016/j.mcn.2014.11.001.
Nanou, E., Sullivan, J. M., Scheuer, T., and Catterall, W. A. (2016) Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons, Proc. Natl. Acad. Sci. USA, 113, 1062-1067, https://doi.org/10.1073/pnas.1524636113.
Mochida, S. (2011) Activity-dependent regulation of synaptic vesicle exocytosis and presynaptic short-term plasticity, Neurosci. Res., 70, 16-23, https://doi.org/10.1016/j.neures.2011.03.005.
Zhao, C., Dreosti, E., and Lagnado, L. (2011) Homeostatic synaptic plasticity through changes in presynaptic calcium influx, J. Neurosci., 31, 7492-7496, https://doi.org/10.1523/JNEUROSCI.6636-10.2011.
Leal, K., Mochida, S., Scheuer, T., and Catterall, W. A. (2012) Fine-tuning synaptic plasticity by modulation of CaV2.1 channels with Ca2+ sensor proteins, Proc. Natl. Acad. Sci. USA, 109, 17069-17074, https://doi.org/10.1073/pnas.1215172109.
Thanawala, M. S., and Regehr, W. G. (2013) Presynaptic calcium influx controls neurotransmitter release in part by regulating the effective size of the readily-releasable pool, J. Neurosci., 33, 4625-4633, https://doi.org/10.1523/JNEUROSCI.4031-12.2013.
Lee, A., Westenbroek, R. E., Haeseleer, F., Palczewski, K., Scheuer, T., et al. (2002) Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1, Nat Neurosci., 5, 210-217, https://doi.org/10.1038/nn805.
Magupalli, V. G., Mochida, S., Yan, J., Jiang, X., Westenbroek, R. E., et al. (2013) Ca2+-independent activation of Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain of CaV2.1 calcium channels, J. Biol. Chem., 288, 4637-4648, https://doi.org/10.1074/jbc.M112.369058.
Funding
The work was supported by the State Assignment of the Ministry of Science and Higher Education of the Russian Federation for 2021-2023 (AAAA-A17-117092040002-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest. The funding organization had no role in the study design, collection, analysis and interpretation of data, writing of the manuscript, and decision to publish the results. All experiments were conducted according to the guidelines of the Declaration of Helsinki, EU Directive 2010/63/EU on the protection of animals used for scientific purposes, and experimental protocols approved by the Ethics Committee of the Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences.
Rights and permissions
About this article
Cite this article
Kudryashova, I. Presynaptic Plasticity Is Associated with Actin Polymerization. Biochemistry Moscow 88, 392–403 (2023). https://doi.org/10.1134/S0006297923030082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923030082