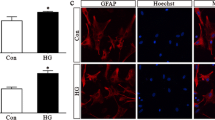
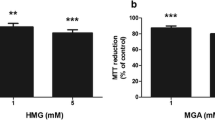
Abstract
Investigation of the relationship between inflammation and energy metabolism is important for understanding biology of chronic noncommunicable diseases. Use of metformin, a drug for treatment of diabetes, is considered as a promising direction for treatment of neurodegenerative diseases and other neuropathologies with an inflammatory component. Astrocytes play an important role in the regulation of energy metabolism and neuroinflammation; therefore, we studied the effect of metformin on the cellular responses of primary rat astrocytes cultured in a medium with high glucose concentration (22.5 mM, 48-h incubation). Lipopolysaccharide (LPS) was used to stimulate inflammation. The effects of metformin were assessed by monitoring changes in the expression of proinflammatory cytokines and synthesis of oxylipins, assayed with ultra-high-performance liquid chromatography and tandem mass spectrometry (UPLC-MS/MS). Changes at the intracellular level were assessed by analyzing phosphorylation of ERK kinase and transcription factor STAT3, as well as enzymes mediating oxylipin synthesis, cyclooxygenase 1 and 2 (COX). It was found that, independent on glucose concentration, metformin reduced the LPS-stimulated release of cytokines IL-1β and IL-6, decreased activity of the transcription factor STAT3, ERK kinase, synthesis of the derivatives of the cyclooxygenase branch of metabolism of oxylipins and anandamide, and did not affect formation of ROS. The study of energy phenotype of the cells showed that metformin activated glycolysis and inhibited mitochondrial respiration and oxidative phosphorylation, independent on LPS stimulation and cell cultivation at high glucose concentration. Thus, it has been shown that metformin exhibits anti-inflammatory effects, and its effect on the synthesis of cytokines, prostaglandins, and other lipid mediators could determine beneficial effects of metformin in models of neuropathology.






Similar content being viewed by others
Abbreviations
- AA:
-
arachidonic acid
- AEA:
-
anandamide acid
- AMPK:
-
5′ AMP-activated protein kinase
- COX:
-
cyclooxygenase
- DHA:
-
docosahexaenoic acid
- ECAR:
-
extracellular acidification rate
- EPA:
-
eicosapentaenoic acid
- HG:
-
high glucose concentration
- IL:
-
interleukin
- LPS:
-
lipopolysaccharide
- NG:
-
normal glucose concentration
- OCR:
-
oxygen consumption rate
- PG:
-
prostaglandin
- ROS:
-
reactive oxygen species
- TNFα:
-
tumor necrosis factor alpha
References
Bai, B., and Chen, H. (2021) Metformin: A novel weapon against inflammation, Front. Pharmacol., 12, 622262, https://doi.org/10.3389/fphar.2021.622262.
Foretz, M., Guigas, B., and Viollet, B. (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus, Nat. Rev. Endocrinol., 15, 569-589, https://doi.org/10.1038/s41574-019-0242-2.
Markowicz-Piasecka, M., Sikora, J., Szydłowska, A., Skupień, A., Mikiciuk-Olasik, E., et al. (2017) Metformin – a future therapy for neurodegenerative diseases, Pharm. Res., 34, 2614-2627, https://doi.org/10.1007/s11095-017-2199-y.
Chistyakov, D. V., Astakhova, A. A., and Sergeeva, M. G. (2018) Resolution of inflammation and mood disorders, Exp. Mol. Pathol., 105, 190-201, https://doi.org/10.1016/j.yexmp.2018.08.002.
Chistyakov, D. V., Goriainov, S. V., Astakhova, A. A., and Sergeeva, M. G. (2021) High glucose shifts the oxylipin profiles in the astrocytes towards pro-inflammatory statesm, Metabolites, 11, 311, https://doi.org/10.3390/metabo11050311.
Kim, S. A., and Choi, H. C. (2012) Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells, Biochem. Biophys. Res. Commun., 425, 866-872, https://doi.org/10.1016/j.bbrc.2012.07.165.
Jing, Y., Wu, F., Li, D., Yang, L., Li, Q., and Li, R. (2018) Metformin improves obesity-associated inflammation by altering macrophages polarization, Mol. Cell. Endocrinol., 461, 256-264, https://doi.org/10.1016/j.mce.2017.09.025.
Qi, T., Chen, Y., Li, H., Pei, Y., Woo, S. L., et al. (2017) A role for PFKFB3/iPFK2 in metformin suppression of adipocyte inflammatory responses, J. Mol. Endocrinol., 59, 49-59, https://doi.org/10.1530/JME-17-0066.
Zhou, J., Massey, S., Story, D., and Li, L. (2018) Metformin: An old drug with new applications, Int. J. Mol. Sci., 19, 2863, https://doi.org/10.3390/ijms19102863.
Escartin, C., Galea, E., Lakatos, A., O’Callaghan, J. P., Petzold, G. C., et al. (2021) Reactive astrocyte nomenclature, definitions, and future directions, Nat. Neurosci., 24, 312-325, https://doi.org/10.1038/s41593-020-00783-4.
Linnerbauer, M., Wheeler, M. A., and Quintana, F. J. (2020) Astrocyte crosstalk in CNS inflammation, Neuron, 108, 608-622, https://doi.org/10.1016/j.neuron.2020.08.012.
Lalo, U., and Pankratov, Y. (2021) Astrocytes as perspective targets of exercise- and caloric restriction-mimetics, Neurochem. Res., 46, 2746-2759, https://doi.org/10.1007/s11064-021-03277-2.
Bélanger, M., Allaman, I., and Magistretti, P. J. (2011) Brain energy metabolism: Focus on Astrocyte-neuron metabolic cooperation, Cell Metab., 14, 724-738, https://doi.org/10.1016/j.cmet.2011.08.016.
Rotermund, C., Machetanz, G., and Fitzgerald, J. C. (2018) The therapeutic potential of metformin in neurodegenerative diseases, Front. Endocrinol. (Lausanne), 9, 400, https://doi.org/10.3389/fendo.2018.00400.
Li, W., Chaudhari, K., Shetty, R., Winters, A., Gao, X., et al. (2019) Metformin alters locomotor and cognitive function and brain metabolism in normoglycemic mice, Aging Dis., 10, 949-963, https://doi.org/10.14336/AD.2019.0120.
Ge, A., Wang, S., Miao, B., and Yan, M. (2018) Effects of metformin on the expression of AMPK and STAT3 in the spinal dorsal horn of rats with neuropathic pain, Mol. Med. Rep., 17, 5229-5237, https://doi.org/10.3892/mmr.2018.8541.
Ou, Z., Kong, X., Sun, X., He, X., Zhang, L., et al. (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice, Brain. Behav. Immun., 69, 351-363, https://doi.org/10.1016/j.bbi.2017.12.009.
Wang, G., Cui, W., Chen, S., Shao, Z., Li, Y., et al. (2021) Metformin alleviates high glucose-induced ER stress and inflammation by inhibiting the interaction between caveolin1 and AMPKα in rat astrocytes, Biochem. Biophys. Res. Commun., 534, 908-913, https://doi.org/10.1016/j.bbrc.2020.10.075.
Hohnholt, M. C., Blumrich, E. M., Waagepetersen, H. S., and Dringen, R. (2017) The antidiabetic drug metformin decreases mitochondrial respiration and tricarboxylic acid cycle activity in cultured primary rat astrocytes, J. Neurosci. Res., 95, 2307-2320, https://doi.org/10.1002/jnr.24050.
Westhaus, A., Blumrich, E. M., and Dringen, R. (2017) The antidiabetic drug metformin stimulates glycolytic lactate production in cultured primary rat astrocytes, Neurochem. Res., 42, 294-305, https://doi.org/10.1007/s11064-015-1733-8.
Astakhova, A., Chistyakov, D., Thomas, D., Geisslinger, G., Brüne, B., et al. (2019) Inhibitors of oxidative phosphorylation modulate astrocyte inflammatory responses through AMPK-dependent Ptgs2 mRNA stabilization, Cells, 8, 1185, https://doi.org/10.3390/cells8101185.
Chistyakov, D. V., Azbukina, N. V., Astakhova, A. A., Polozhintsev, A. I., Sergeeva, M. G., et al. (2019) Toll-like receptors control p38 and JNK MAPK signaling pathways in rat astrocytes differently, when cultured in normal or high glucose concentrations, Neurochem. Int., 131, 104513, https://doi.org/10.1016/j.neuint.2019.104513.
Chistyakov, D. V., Gavrish, G. E., Goriainov, S. V., Chistyakov, V. V., Astakhova, A. A., et al. (2020) Oxylipin profiles as functional characteristics of acute inflammatory responses in astrocytes pre-treated with IL-4, IL-10, or LPS, Int. J. Mol. Sci., 21, 1780, https://doi.org/10.3390/ijms21051780.
Chistyakov, D. V., Aleshin, S. E., Astakhova, A. A., Sergeeva, M. G., and Reiser, G. (2015) Regulation of peroxisome proliferator-activated receptors (PPAR) α and -γ of rat brain astrocytes in the course of activation by toll-like receptor agonists, J. Neurochem., 134, 113-124, https://doi.org/10.1111/jnc.13101.
Guryleva, M. V., Chistyakov, D. V., Lopachev, A. V., Goriainov, S. V., Astakhova, A. A., et al. (2021) Modulation of the primary astrocyte-enriched cultures’ oxylipin profiles reduces neurotoxicity, Metabolites, 11, 498, https://doi.org/10.3390/metabo11080498.
Gong, P., Xu, X., Shi, J., Ni, L., Huang, Q., et al. (2013) Phosphorylation of mitogen- and stress-activated protein kinase-1 in astrocytic inflammation: A possible role in inhibiting production of inflammatory cytokines, PLoS One, 8, e81747, https://doi.org/10.1371/journal.pone.0081747.
Liu, X., Tian, Y., Lu, N., Gin, T., Cheng, C. H. K., et al. (2013) Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes, PLoS One, 8, e75804, https://doi.org/10.1371/journal.pone.0075804.
Kim, J., Kwak, H. J., Cha, J. Y., Jeong, Y. S., Rhee, S. D., et al. (2014) Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via Activating Transcription Factor-3 (ATF-3) induction, J. Biol. Chem., 289, 23246-23255, https://doi.org/10.1074/jbc.M114.577908.
Li, W., Choudhury, G. R., Winters, A., Prah, J., Lin, W., et al. (2018) Hyperglycemia alters astrocyte metabolism and inhibits astrocyte proliferation, Aging Dis., 9, 674-684, https://doi.org/10.14336/AD.2017.1208.
Quincozes-Santos, A., Bobermin, L. D., de Assis, A. M., Gonçalves, C. A., and Souza, D. O. (2017) Fluctuations in glucose levels induce glial toxicity with glutamatergic, oxidative and inflammatory implications, Biochim. Biophys. Acta Mol. Basis Dis., 1863, 1-14, https://doi.org/10.1016/j.bbadis.2016.09.013.
Wang, J., Li, G., Wang, Z., Zhang, X., Yao, L., et al. (2012) High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes, Neuroscience, 202, 58-68, https://doi.org/10.1016/j.neuroscience.2011.11.062.
Xian, H., Liu, Y., Rundberg Nilsson, A., Gatchalian, R., Crother, T. R., et al. (2021) Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation, Immunity, 54, 1463-1477.e11, https://doi.org/10.1016/j.immuni.2021.05.004.
Tayara, K., Espinosa-Oliva, A. M., García-Domínguez, I., Ismaiel, A. A., Boza-Serrano, A., et al. (2018) Divergent effects of metformin on an inflammatory model of Parkinson’s disease, Front. Cell. Neurosci., 12, 440, https://doi.org/10.3389/fncel.2018.00440.
Beurel, E., and Jope, R. S. (2009) Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain, J. Neuroinflamm., 6, 9, https://doi.org/10.1186/1742-2094-6-9.
Lamichhane, S., Bastola, T., Pariyar, R., Lee, E. S., Lee, H. S., et al. (2017) ROS production and ERK activity are involved in the effects of D-β-hydroxybutyrate and metformin in a glucose deficient condition, Int. J. Mol. Sci., 18, 674, https://doi.org/10.3390/ijms18030674.
De Morais, H., de Souza, C. P., da Silva, L. M., Ferreira, D. M., Baggio, C. H., et al. (2016) Anandamide reverses depressive-like behavior, neurochemical abnormalities and oxidative-stress parameters in streptozotocin-diabetic rats: role of CB1 receptors, Eur. Neuropsychopharmacol., 26, 1590-1600, https://doi.org/10.1016/j.euroneuro.2016.08.007.
Cui, N., Feng, X., Zhao, Z., Zhang, J., Xu, Y., et al. (2017) Restored plasma anandamide and endometrial expression of fatty acid amide hydrolase in women with polycystic ovary syndrome by the combination use of Diane-35 and metformin, Clin. Ther., 39, 751-758, https://doi.org/10.1016/j.clinthera.2017.02.007.
Quan, Y., Jiang, C. T., Xue, B., Zhu, S. G., and Wang, X. (2011) High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways, Acta Pharmacol. Sin., 32, 188-193, https://doi.org/10.1038/aps.2010.174.
Qiu, Z., He, Y., Ming, H., Lei, S., Leng, Y., et al. (2019) Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-Dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes, J. Diabetes Res., 2019, 8151836, https://doi.org/10.1155/2019/8151836.
Kajiwara, C., Kusaka, Y., Kimura, S., Yamaguchi, T., Nanjo, Y., et al. (2018) Metformin mediates protection against legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species, J. Immunol., 200, 623-631, https://doi.org/10.4049/jimmunol.1700474.
Barros, L. F., Ruminot, I., San Martín, A., Lerchundi, R., Fernández-Moncada, I., et al. (2021) Aerobic glycolysis in the brain: Warburg and Crabtree Contra Pasteur, Neurochem. Res., 46, 15-22, https://doi.org/10.1007/s11064-020-02964-w.
Zhang, J., Zhang, L., Nie, J., Lin, Y., Li, Y., et al. (2021) Calcineurin inactivation inhibits pyruvate dehydrogenase complex activity and induces the Warburg effect, Oncogene, 40, 6692-6702, https://doi.org/10.1038/s41388-021-02065-0.
Alhourani, A. H., Tidwell, T. R., Bokil, A. A., Røsland, G. V., Tronstad, K. J., et al. (2021) Metformin treatment response is dependent on glucose growth conditions and metabolic phenotype in colorectal cancer cells, Sci. Rep., 11, 10487, https://doi.org/10.1038/s41598-021-89861-6.
Frauwirth, K. A., Riley, J. L., Harris, M. H., Parry, R. V., Rathmell, J. C., et al. (2002) The CD28 signaling pathway regulates glucose metabolism, Immunity, 16, 769-777, https://doi.org/10.1016/s1074-7613(02)00323-0.
Mason, S. (2017) Lactate shuttles in neuroenergetics-homeostasis, allostasis and beyond, Front. Neurosci., 11, 43, https://doi.org/10.3389/fnins.2017.00043.
Acknowledgments
The authors are grateful to the Moscow State University Development Program for providing access to the confocal microscope Zeiss LSM900.
Funding
This study was financially supported by the Russian Science Foundation (project no. 20-74-00068).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. All applicable international, national, and/or institutional guidelines for the care and use of animals were
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gorbatenko, V.O., Goriainov, S.V., Babenko, V.A. et al. Anti-Inflammatory Properties of Metformin During Cultivation of Primary Rat Astrocytes in a Medium with High Glucose Concentration. Biochemistry Moscow 87, 577–589 (2022). https://doi.org/10.1134/S000629792207001X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792207001X