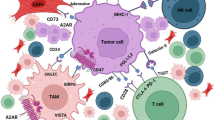
Abstract
Undoubtedly, one of the most promising approaches to the treatment of cancer is creation of the pathogenetically based therapeutic drugs. Researchers from all over the world are trying to answer the question on how to select a target that would be effective and, in general, they are quite successful at that. The Nobel Prize-winning discovery of mechanisms for regulating activity of the immune system cells through checkpoint molecules, as well as discovery of the ability of tumor cells to use these mechanisms to suppress immune responses was an impetus for the development of modern immunotherapy, and now such inhibitors of the immune checkpoints as PD-1/PD-L1 are included in the routine chemotherapy. Use of such drugs can prolong the patient’s life, but, unfortunately, not cure the disease. This is partially due to heterogeneity of tumor cells and microenvironment, but the main reasons may be in the complex relationships between the tumor and microenvironment, which, at times, are so plastic that they can change, adjusting to newly emerging conditions. Main characteristic of the tumor microenvironment is the type of the ongoing immune-inflammatory response (IIR), and since inhibitors of the immune checkpoints act on the cells involved in IIR, it is obvious that the outcomes of cancer therapy, including outcomes of hyperprogressive disease, can be associated with this parameter. The presented review reveals the essence of interactions between the tumor and its microenvironment during therapy with PD-L1 inhibitors.
Similar content being viewed by others
Abbreviations
- BC:
-
breast carcinoma
- CTLA-4:
-
cytotoxic T-lymphocyte-associated protein 4
- HPD:
-
hyperprogressive disease
- IIR:
-
immune-inflammatory response
- IL:
-
interleukin
- NF-κB:
-
nuclear factor kappa-B
- NK cells:
-
natural killer cells
- NSCLC:
-
non-small cell lung cancer
- PD-1:
-
programmed cell death protein 1
- PD-L1:
-
programmed cell death ligand 1
- TILs:
-
tumor-infiltrating lymphocytes
- TNF-α:
-
tumor necrosis factor-α
References
Zak, K. M., Grudnik, P., Magiera, K., Dömling, A., Dubin, G., and Holak, T. A. (2017) Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2, Structure, 25, 1163-1174, https://doi.org/10.1016/j.str.2017.06.011.
Butte, M. J., Keir, M. E., Phamduy, T. B., Sharpe, A. H., and Freeman, G. J. (2007) Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses, Immunity, 27, 111-122, https://doi.org/10.1016/j.immuni.2007.05.016.
Dong, H., Strome, S. E., Salomao, D. R., Tamura, H., Hirano, F., et al. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion, Nat. Med., 8, 793-800, https://doi.org/10.1038/nm730.
Gatalica, Z., Snyder, C., Maney, T., Ghazalpour, A., Holterman, D. A., et al. (2014) Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type, Cancer Epidemiol. Biomarkers Prevention, 23, 2965-2970, https://doi.org/10.1158/1055-9965.EPI-14-0654.
Bardhan, K., Anagnostou, T., and Boussiotis, V. A. (2016) The PD1:PD-L1/2 pathway from discovery to clinical implementation, Front. Immunol., 7, 500, https://doi.org/10.3389/fimmu.2016.00550.
Yearley, J. H., Gibson, C., Yu, N., Moon, C., Murphy, E., et al. (2017) PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer, Clin. Cancer Res., 23, 3158-3167, https://doi.org/10.1158/1078-0432.CCR-16-1761.
Singh, A.K., Stock, P., Akbari, O. (2011) Role of PD-L1 and PD-L2 in allergic diseases and asthma, Allergy, 66, 155-162.
Ghosh, C., Luong, G., and Sun, Y. (2021) A snapshot of the PD-1/PD-L1 pathway, J. Cancer, 12, 2735-2746, https://doi.org/10.7150/jca.57334.
Buchbinder, E. I., and Desai, A. (2016) CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition, Am. J. Clin. Oncol., 39, 98-106, https://doi.org/10.1097/COC.0000000000000239.
Ghiotto, M., Gauthier, L., Serriari, N., Pastor, S., Truneh, A., et al. (2010) PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1, Int. Immunol., 22, 651-660, https://doi.org/10.1093/intimm/dxq049.
Arrieta, O., Montes-Servín, E., Hernandez-Martinez, J.-M., Cardona, A. F., Casas-Ruiz, E., et al. (2017) Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients, Oncotarget, 8, 101994-102005, https://doi.org/10.18632/oncotarget.22025.
Zhu, S., Lin, J., Qiao, G., Wang, X., and Xu, Y. (2016) Tim-3 identifies exhausted follicular helper T cells in breast cancer patients, Immunobiology, 221, 986-993, https://doi.org/10.1016/j.imbio.2016.04.005.
Sharma, P., Siddiqui, B. A., Anandhan, S., Yadav, S. S., Subudhi, S. K., et al. (2021) The next decade of immune checkpoint therapy, Cancer Discov., 11, 838-857, https://doi.org/10.1158/2159-8290.CD-20-1680.
Patel, S. P., and Kurzrock, R. (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy, Mol. Cancer Ther., 14, 847-856, https://doi.org/10.1158/1535-7163.MCT-14-0983.
Powles, T., Eder, J. P., Fine, G. D., Braiteh, F. S., Loriot, Y., et al. (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer, Nature, 515, 558-562, https://doi.org/10.1038/nature13904.
Ai, L., Chen, J., Yan, H., He, Q., Luo, P., et al. (2020) Research status and outlook of PD-1/PD-L1 inhibitors for cancer therapy, Drug Des. Dev. Ther., 14, 3625-3649, https://doi.org/10.2147/DDDT.S267433.
Teng, F., Meng, X., Kong, L., and Yu, J. (2018) Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review, Cancer Lett., 414, 166-173, https://doi.org/10.1016/j.canlet.2017.11.014.
Teng, M. W., Ngiow, S. F., Ribas, A., and Smyth, M. J. (2015) Classifying cancers based on T-cell infiltration and PD-L1, Cancer Res., 75, 2139-2145, https://doi.org/10.1158/0008-5472.CAN-15-0255.
Emens, L. A., Cruz, C., Eder, J. P., Braiteh, F., Chung, C., et al. (2019) Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study, JAMA Oncol., 5, 74-82, https://doi.org/10.1001/jamaoncol.2018.4224.
Tashireva, L. A., Perelmuter, V. M., Denisov, E. V., Savelieva, O. E., et al. (2017) Types of immune-inflammatory responses as a reflection of cell–cell interactions under conditions of tissue regeneration and tumor growth, Biochemistry (Moscow), 82, 542-555, https://doi.org/10.1134/S0006297917050029.
Perelmuter, V. M., Tashireva, L. A., Manskikh, V. N., Denisov, E. V., Savelieva, O. E., et al. (2017) Heterogeneity and plasticity of immune-inflammatory responses in tumor microenvironment: A role in antitumor effect and tumor aggressiveness, Zhurn. Obsch. Biol., 78, 15-36.
Emens, L., Loi, S., Rugo, H., Schneeweiss, A., Diéras, V., et al. (2019) Abstract GS1-04: IMpassion130: Efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab + nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer, Cancer Res., 79, GS1-04, https://doi.org/10.1158/1538-7445.SABCS18-GS1-04.
Catacchio, I., Silvestris, N., Scarpi, E., Schirosi, L., Scattone, A., and Mangia, A. (2019) Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients, Transl. Oncol., 12, 585-595, https://doi.org/10.1016/j.tranon.2018.12.005.
Somasundaram, R., Connelly, T., Choi, R., Choi, H., Samarkina, A., et al. (2021) Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy, Nat. Commun., 12, 1-14, https://doi.org/10.1038/s41467-020-20600-7.
Abiko, K., Matsumura, N., Hamanishi, J., Horikawa, N., Murakami, R., et al. (2015) IFN-α from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer, Br. J. Cancer, 112, 1501-1509.
Sun, C., Mezzadra, R., and Schumacher, T. N. (2018) Regulation and function of the PD-L1 checkpoint, Immunity, 48, 434-452, https://doi.org/10.1016/j.immuni.2018.03.014.
Shi, Y. (2018) Regulatory mechanisms of PD-L1 expression in cancer cells, Cancer Immunol. Immunother., 67, 1481-1489, https://doi.org/10.1007/s00262-018-2226-9.
Yamazaki, T., Akiba, H., Iwai, H., Matsuda, H., Aoki, M., et al. (2002) Expression of programmed death 1 ligands by murine T cells and APC, J. Immunol., 169, 5538-5545, https://doi.org/10.4049/jimmunol.169.10.5538.
Loke, P., and Allison, J. P. (2003) PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells, Proc. Natl. Acad. Sci. USA, 100, 5336-5341.
Wang, X., Teng, F., Kong, L., and Yu, J. (2016) PD-L1 expression in human cancers and its association with clinical outcomes, Onco Targets Ther., 9, 5023-5039, https://doi.org/10.2147/OTT.S105862.
Budczies, J., Bockmayr, M., Denkert, C., Klauschen, F., Gröschel, S., et al. (2016) Pan-cancer analysis of copy number changes in programmed death-ligand 1 (PD-L1, CD274)-associations with gene expression, mutational load, and survival, Genes Chromosomes Cancer, 55, 626-639, https://doi.org/10.1002/gcc.22365.
Mittendorf, E. A., Philips, A. V., Meric-Bernstam, F., Qiao, N., Wu, Y., et al. (2014) PD-L1 expression in triple-negative breast cancer, Cancer Immunol. Res., 2, 361-370, https://doi.org/10.1158/2326-6066.CIR-13-0127.
Noman, M. Z., Desantis, G., Janji, B., Hasmim, M., Karray, S., et al. (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation, J. Exp. Med., 211, 781-790, https://doi.org/10.1084/jem.20131916.
Shehade, H., Oldenhove, G., and Moser, M. (2014) Hypoxia in the intestine or solid tumors: a beneficial or deleterious alarm signal, Eur. J. Immunol., 44, 2550-2557, https://doi.org/10.1002/eji.201444719.
Dong, H., Zhu, G., Tamada, K., and Chen, L. (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion, Nat. Med., 5, 1365-1369, https://doi.org/10.1038/70932.
Carter, L., Fouser, L. A., Jussif, J., Fitz, L., Deng, B., et al. (2002) PD‐1:PD‐L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2, Eur. J. Immunol., 32, 634-643, https://doi.org/10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9.
Freeman, G. J., Long, A. J., Iwai, Y., Bourque, K., Chernova, T., et al. (2000) Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation, J. Exp. Med., 192, 1027-1034, https://doi.org/10.1084/jem.192.7.1027.
Karyampudi, L., Lamichhane, P., Krempski, J., Kalli, K. R., Behrens, M. D., et al. (2016) PD‐1 blunts the function of ovarian tumor‐infiltrating dendritic cells by inactivating NF-κB, Cancer Res., 76, 239-250, https://doi.org/10.1158/0008-5472.CAN-15-0748.
Gettinger, S. N., Choi, J., Mani, N., Sanmamed, M. F., Datar, I., et al. (2018) A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers, Nat. Commun., 9, 1-15, https://doi.org/10.1038/s41467-018-05032-8.
Solaymani-Mohammadi, S., Lakhdari, O., Minev, I., Shenouda, S., Frey, B. F., et al. (2016) Lack of the programmed death-1 receptor renders host susceptible to enteric microbial infection through impairing the production of the mucosal natural killer cell effector molecules, J. Leukoc. Biol., 99, 2-9, https://doi.org/10.1189/jlb.4A0115-003RR.
Niu, C., Li, M., Zhu, S., Chen, Y., Zhou, L., et al. (2020) PD-1-positive Natural Killer Cells have a weaker antitumor function than that of PD-1-negative Natural Killer Cells in lung cancer, Int. Med. Sci., 17, 1964-1973, https://doi.org/10.7150/ijms.47701.
Lamichhane, P., Karyampudi, L., Shreeder, B., Krempski, J., Bahr, D., et al. (2017) IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer, Cancer Res., 77, 6667-6678, https://doi.org/10.1158/0008-5472.CAN-17-0740.
Yamaguchi, K., Mishima, K., Ohmura, H., Hanamura, F., Ito, M., et al. (2018) Activation of central/effector memory T cells and Th1 polarization in malignant melanoma patients treated with anti-PD-1 antibody, Cancer Sci., 109, 3032-3042, https://doi.org/10.1111/cas.13758.
Dulos, J., Carven, G. J., van Boxtel, S. J., Evers, S., Driessen-Engels, L. J., et al. (2012) PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer, J. Immunother., 35, 169-178, https://doi.org/10.1097/CJI.0b013e318247a4e7.
Tavukcuoglu, E., Horzum, U., Yilmaz, K. B., and Esendagli, G. (2020) PD-L2+ wound zone macrophage-like cells display M1/M2-mixed activation and restrain the effector Th1 responses, Immunol. Cell Biol., 98, 152-164, https://doi.org/10.1111/imcb.12310.
Verma, V., Shrimali, R. K., Ahmad, S., Dai, W., Wang, H., et al. (2019) PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance, Nat. Immunol., 20, 1231-1243, https://doi.org/10.1038/s41590-019-0441-y.
Chubachi, S., Yasuda, H., Irie, H., Fukunaga, K., Naoki, K., et al. (2016) A case of non‐small cell lung cancer with possible “disease flare” on nivolumab treatment, Case Rep., 2016, 1-3, https://doi.org/10.1155/2016/1075641.
Champiat, S., Dercle, L., Ammari, S., Massard, C., Hollebecque, A., et al. (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1, Clin. Cancer Res., 23, 1920‐1928, https://doi.org/10.1158/1078-0432.CCR-16-1741.
Lahmar, J., Mezquita, L., Koscielny, S., Facchinetti, F., Bluthgen, M. V., et al. (2016) Immune checkpoint inhibitors (IC) induce paradoxical progression in a subset of non-small cell lung cancer (NSCLC), Ann. Oncol., 27, vi423, https://doi.org/10.1093/annonc/mdw383.22.
Ferrara, R., Mezquita, L., Texier, M., Lahmar, J., Audigier-Valette, C., et al. (2018) Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy, JAMA Oncol., 4, 1543-1552, https://doi.org/10.1001/jamaoncol.2018.3676.
Kato, S., Goodman, A., Walavalkar, V., Barkauskas, D. A., Sharabi, A., and Kurzrock, R. (2017) Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate, Clin. Cancer Res., 15, 4242-4250, https://doi.org/10.1158/1078-0432.CCR-16-3133.
Saâda-Bouzid, E., Defaucheux, C., Karabajakian, A., Coloma, V. P., Servois, V., et al. (2017) Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma, Ann. Oncol., 28, 1605-1611, https://doi.org/10.1093/annonc/mdx178.
Kim, C. G., Kim, K. H., Pyo, K. H., Xin, C. F., Hong, M. H., et al. (2019) Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer, Ann. Oncol., 30, 1104-1113, https://doi.org/10.1093/annonc/mdz123.
Lo Russo, G., Moro, M., Sommariva, M., Cancila, V., Boeri, M., et al. (2019) Antibody-fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade, Clin. Cancer Res., 25, 989-999, https://doi.org/10.1158/1078-0432.CCR-18-1390.
Kamada, T., Togashi, Y., Tay, C., Ha, D., Sasaki, A., et al. (2019) PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer, Proc. Natl. Acad. Sci. USA, 116, 9999-10008, https://doi.org/10.1073/pnas.1822001116.
Arasanz, H., Zuazo, M., Bocanegra, A., Gato, M., Martínez-Aguillo, M., et al. (2020) Early detection of hyperprogressive disease in non‐small cell lung cancer by monitoring of systemic T cell dynamics, Cancers (Basel), 12, 334, https://doi.org/10.3390/cancers12020344.
Kim, S. R., Chun, S. H., Kim, J. R., Kim, S.-Y., Seo, J. Y., et al. (2021) The implications of clinical risk factors, CAR index, and compositional changes of immune cells on hyperprogressive disease in non-small cell lung cancer patients receiving immunotherapy, BMC Cancer, 21, 1-11, https://doi.org/10.1186/s12885-020-07727-y.
Sun, Z., Fourcade, J., Pagliano, O., Chauvin, J.-M., Sander, C., et al. (2015) IL10 and PD‐1 cooperate to limit the activity of tumor‐specific CD8+ T cells, Cancer Res., 75, 1635-1644, https://doi.org/10.1158/0008-5472.CAN-14-3016.
Groux, H., Bigler, M., de Vries, J. E., and Roncarolo, M. G. (1996) Interleukin-10 induces a long‐term antigen‐specific anergic state in human CD4+ T cells, J. Exp. Med., 184, 19‐29, https://doi.org/10.1084/jem.184.1.19.
O’Garra, A., Barrat, F. J., Castro, A. G., Vicari, A., and Hawrylowicz, C. (2008) Strategies for use of IL-10 or its antagonists in human disease, Immunol. Rev., 223, 114-131, https://doi.org/10.1111/j.1600-065X.2008.00635.x.
Gato-Cañas, M., Zuazo, M., Arasanz, H., Ibañez-Vea, M., Lorenzo, L., et al. (2017) PD-L1 signals through conserved sequence motifs to overcome interferon-mediated cytotoxicity, Cell Rep., 20, 1818-1829, https://doi.org/10.1016/j.celrep.2017.07.075.
Xiong, D., Wang, Y., Singavi, A. K., Mackinnon, A. C., George, B., and You, M. (2018) Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer, iScience, 9, 258-277, https://doi.org/10.1016/j.isci.2018.10.021.
Stathopoulou, C., Gangaplara, A., Mallett, G., Flomerfelt, F. A., Liniany, L. P., et al. (2018) PD-1 Inhibitory receptor downregulates asparaginyl endopeptidase and maintains Foxp3 transcription factor stability in induced regulatory T cells, Immunity, 49, 247-263, https://doi.org/10.1016/j.immuni.2018.05.006.
Tsukamoto, H., Fujieda, K., Miyashita, A., Fukushima, S., Ikeda, T., et al. (2018) Combined blockade of IL-6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment, Cancer Res., 78, 5011-5022, https://doi.org/10.1158/0008-5472.CAN-18-0118.
Schwartz, C., Khan, A. R., Floudas, A., Saunders, S. P., Hams, E., et al. (2017) ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control, J. Exp. Med., 214, 2507-2521, https://doi.org/10.1084/jem.20170051.
Funding
This work was financially supported by the Russian Science Foundation (project no. 20-75-10033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tashireva, L.A., Muravyova, D.T., Popova, N.O. et al. Parameters of Tumor Microenvironment Determine Effectiveness of Anti-PD-1/PD-L1 Therapy. Biochemistry Moscow 86, 1461–1468 (2021). https://doi.org/10.1134/S0006297921110092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921110092