Abstract
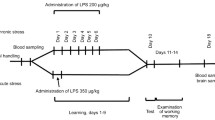
According to the two-hit hypothesis of psychoneuropathology formation, infectious diseases and other pathological conditions occurring during the critical periods of early ontogenesis disrupt normal brain development and increase its susceptibility to stress experienced in adolescence and adulthood. It is believed that these disorders are associated with changes in the functional activity of the glutamatergic system in the hippocampus. Here, we studied expression of NMDA (GluN1, GluN2a, GluN2b) and AMPA (GluA1, GluA2) glutamate receptor subunits, as well as glutamate transporter EAAT2, in the ventral and dorsal regions of the hippocampus of rats injected with LPS during the third postnatal week and then subjected to predator stress (contact with a python) in adulthood. The tests were performed 25 days after the stress. It was found that stress altered protein expression in the ventral, but not in the dorsal hippocampus. Non-stressed LPS-treated rats displayed lower levels of the GluN2b protein in the ventral hippocampus vs. control animals. Stress significantly increased the content of GluN2b in the LPS-treated rats, but not in the control animals. Stress also affected differently the exploratory behavior of LPS-injected and control rats. Compared to the non-stressed animals, stressed control rats demonstrated a higher locomotor activity during the 1st min of the open field test, while the stressed LPS-injected rats displayed lower locomotor activity than the non-stressed rats. In addition, LPS-treated stressed and non-stressed rats spent more time in the open arms of the elevated plus maze and demonstrated reduced blood levels of corticosterone. To summarize the results of our study, exposure to bacterial LPS in the early postnatal ontogenesis affects the pattern of stress-induced changes in the behavior and hippocampal expression of genes coding for ionotropic glutamate receptor subunits after psychogenic trauma suffered in adulthood.







Similar content being viewed by others
Abbreviations
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- EAAT2:
-
excitatory amino acid transporter 2
- EPMT:
-
elevated plus maze test
- LPS:
-
lipopolysaccharide, endotoxin
- NMDA:
-
N-methyl-D-aspartate
- OFT:
-
open field test
- PTSD:
-
post-traumatic stress disorder
References
Lee, R. S., Oswald, L. M., and Wand, G. S. (2018) Early life stress as a predictor of co-occurring alcohol use disorder and post-traumatic stress disorder, Alcohol. Res., 39, 147-159.
Fisher, P. A., Beauchamp, K. G., Roos, L. E., Noll, L. K., Flannery, J., and Delker, B. C. (2016) The neurobiology of intervention and prevention in early adversity, Annu. Rev. Clin. Psychol., 12, 331-357, https://doi.org/10.1146/annurev-clinpsy-032814-112855.
Van Camp, G., Cigalotti, J., Bouwalerh, H., Mairesse, J., Gatta, E., et al. (2018) Consequences of a double hit of stress during the perinatal period and midlife in female rats: mismatch or cumulative effect? Psychoneuroendocrinology, 93, 45-55, https://doi.org/10.1016/j.psyneuen.2018.04.004.
Jaric, I., Rocks, D., Cham, H., Herchek, A., and Kundakovic, M. (2019) Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model, Front. Mol. Neurosci., 12, 74, https://doi.org/10.3389/fnmol.2019.00074.
Koss, K. J., and Gunnar, M. R. (2018) Annual research review: early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology, J. Child Psychol. Psychiatry, 59, 327-346, https://doi.org/10.1111/jcpp.12784.
Dinel, A.-L., Joffre, C., Trifilieff, P., Aubert, A., Foury, A., Le Ruyet, P., and Layé, S. (2014) Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood, J. Neuroinflammation, 11, 155, https://doi.org/10.1186/s12974-014-0155-x.
Doenni, V. M., Song, C. M., Hill, M. N., and Pittman, Q. J. (2017) Early-life inflammation with LPS delays fear extinction in adult rodents, Brain. Behav. Immun., 63, 176-185, https://doi.org/10.1016/j.bbi.2016.11.022.
Lei, Y., Chen, C.-J., Yan, X.-X., Li, Z., and Deng, X.-H. (2017) Early-life lipopolysaccharide exposure potentiates forebrain expression of NLRP3 inflammasome proteins and anxiety-like behavior in adolescent rats, Brain Res., 1671, 43-54, https://doi.org/10.1016/j.brainres.2017.06.014.
Trofimov, A., Strekalova, T., Mortimer, N., Zubareva, O., Schwarz, A., et al. (2017) Postnatal LPS challenge impacts escape learning and expression of plasticity factors Mmp9 and Timp1 in rats: effects of repeated training, Neurotox. Res., 32, 175-186, https://doi.org/10.1007/s12640-017-9720-2.
Trofimov, A. N., Rotov, A. Y., Veniaminova, E. A., Fomalont, K., Schwarz, A. P., and Zubareva, O. E. (2020) Changes in behavior and the expression of ionotropic glutamate receptor genes in the brains of adult rats after neonatal administration of bacterial lipopolysaccharide, Neurosci. Behav. Physiol., 50, 1239-1248, https://doi.org/10.1007/s11055-020-01025-7.
Zubareva, O. E., Postnikova, T. Y., Grifluk, A. V., Schwarz, A. P., Smolensky, I. V., et al. (2020) Exposure to bacterial lipopolysaccharide in early life affects the expression of ionotropic glutamate receptor genes and is accompanied by disturbances in long-term potentiation and cognitive functions in young rats, Brain. Behav. Immun., 90, 3-15, https://doi.org/10.1016/j.bbi.2020.07.034.
Walker, A. K., Nakamura, T., Byrne, R. J., Naicker, S., Tynan, R. J., et al. (2009) Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis, Psychoneuroendocrinology, 34, 1515-1525, https://doi.org/10.1016/j.psyneuen.2009.05.010.
Walker, A. K., Nakamura, T., and Hodgson, D. M. (2010) Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats, Stress, 13, 506-515, https://doi.org/10.3109/10253890.2010.489977.
Harré, E.-M., Galic, M. A., Mouihate, A., Noorbakhsh, F., and Pittman, Q. J. (2008) Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain, Eur. J. Neurosci., 27, 644-653, https://doi.org/10.1111/j.1460-9568.2008.06031.x.
Hansen, K. B., Yi, F., Perszyk, R. E., Furukawa, H., Wollmuth, L. P., et al. (2018) Structure, function, and allosteric modulation of NMDA receptors, J. Gen. Physiol., 150, 1081-1105, https://doi.org/10.1085/jgp.201812032.
Liu, S., Lau, L., Wei, J., Zhu, D., Zou, S., et al. (2004) Expression of Ca2+-permeable AMPA receptor channels primes cell death in transient forebrain ischemia, Neuron, 43, 43-55, https://doi.org/10.1016/j.neuron.2004.06.017.
Wenzel, A., Fritschy, J. M., Mohler, H., and Benke, D. (1997) NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins, J. Neurochem., 68, 469-478, https://doi.org/10.1046/j.1471-4159.1997.68020469.x.
Babb, T. L., Mikuni, N., Najm, I., Wylie, C., Olive, M., et al. (2005) Pre- and postnatal expressions of NMDA receptors 1 and 2B subunit proteins in the normal rat cortex, Epilepsy Res., 64, 23-30, https://doi.org/10.1016/j.eplepsyres.2005.02.008.
Du Bois, T. M., and Huang, X.-F. (2007) Early brain development disruption from NMDA receptor hypofunction: relevance to schizophrenia, Brain Res. Rev., 53, 260-270, https://doi.org/10.1016/j.brainresrev.2006.09.001.
Lippman-Bell, J. J., Zhou, C., Sun, H., Feske, J. S., and Jensen, F. E. (2016) Early-life seizures alter synaptic calcium-permeable AMPA receptor function and plasticity, Mol. Cell. Neurosci., 76, 11-20, https://doi.org/10.1016/j.mcn.2016.08.002.
Yuan, T., and Bellone, C. (2013) Glutamatergic receptors at developing synapses: the role of GluN3A-containing NMDA receptors and GluA2-lacking AMPA receptors, Eur. J. Pharmacol., 719, 107-111, https://doi.org/10.1016/j.ejphar.2013.04.056.
Sweatt, J. D. (2016) Neural plasticity and behavior – sixty years of conceptual advances, J. Neurochem., 139, 179-199, https://doi.org/10.1111/jnc.13580.
Diering, G. H., and Huganir, R. L. (2018) The AMPA receptor code of synaptic plasticity, Neuron, 100, 314-329, https://doi.org/10.1016/j.neuron.2018.10.018.
Lisman, J. (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling, Philos. Trans. R. Soc. Lond. B Biol. Sci., 372, https://doi.org/10.1098/rstb.2016.0260.
Sarantis, K., Antoniou, K., Matsokis, N., and Angelatou, F. (2012) Exposure to novel environment is characterized by an interaction of D1/NMDA receptors underlined by phosphorylation of the NMDA and AMPA receptor subunits and activation of ERK1/2 signaling, leading to epigenetic changes and gene expression in rat hippocampus, Neurochem. Int., 60, 55-67, https://doi.org/10.1016/j.neuint.2011.10.018.
Barkus, C., McHugh, S. B., Sprengel, R., Seeburg, P. H., Rawlins, J. N. P., and Bannerman, D. M. (2010) Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion, Eur. J. Pharmacol., 626, 49-56, https://doi.org/10.1016/j.ejphar.2009.10.014.
Pacheco, A., Aguayo, F. I., Aliaga, E., Muñoz, M., García-Rojo, G., et al. (2017) Chronic stress triggers expression of immediate early genes and differentially affects the expression of AMPA and NMDA subunits in dorsal and ventral hippocampus of rats, Front. Mol. Neurosci., 10, 244, https://doi.org/10.3389/fnmol.2017.00244.
Costa-Nunes, J., Zubareva, O., Araújo-Correia, M., Valença, A., Schroeter, C. A., et al. (2014) Altered emotionality, hippocampus-dependent performance and expression of NMDA receptor subunit mRNAs in chronically stressed mice, Stress, 17, 108-116, https://doi.org/10.3109/10253890.2013.872619.
Furini, C., Myskiw, J., and Izquierdo, I. (2014) The learning of fear extinction, Neurosci. Biobehav. Rev., 47, 670-683, https://doi.org/10.1016/j.neubiorev.2014.10.016.
Matsumoto, Y., Morinobu, S., Yamamoto, S., Matsumoto, T., Takei, S., et al. (2013) Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder, Psychopharmacology (Berlin), 229, 51-62, https://doi.org/10.1007/s00213-013-3078-9.
Réus, G. Z., Abelaira, H. M., Stringari, R. B., Fries, G. R., Kapczinski, F., and Quevedo, J. (2012) Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats, Metab. Brain Dis., 27, 175-182, https://doi.org/10.1007/s11011-012-9281-2.
Padovan, C. M., and Guimarães, F. S. (2004) Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats, Pharmacol. Biochem. Behav., 77, 15-19, https://doi.org/10.1016/j.pbb.2003.09.015.
Blacker, C. J., Millischer, V., Webb, L. M., Ho, A. M. C., Schalling, M., et al. (2020) EAAT2 as a research target in bipolar disorder and unipolar depression: a systematic review, Mol. Neuropsychiatry, 5, 44-59, https://doi.org/10.1159/000501885.
Zink, M., Vollmayr, B., Gebicke-Haerter, P. J., and Henn, F. A. (2010) Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression, Neuropharmacology, 58, 465-473, https://doi.org/10.1016/j.neuropharm.2009.09.005.
Zhang, X. H., Jia, N., Zhao, X. Y., Tang, G. K., Guan, L. X., et al. (2013) Involvement of pGluR1, EAAT2 and EAAT3 in offspring depression induced by prenatal stress, Neuroscience, 250, 333-341, https://doi.org/10.1016/j.neuroscience.2013.04.031.
Kovalenko, A. A., Zakharova, M. V., Nikitina, V. A., Schwarz, A. P., Karyakin, V. B., et al. (2018) Alterations in the expression of genes that encode subunits of ionotropic glutamate receptors and the glutamate transporter in brain structures of rats after psychogenic stress, Neurochem. J., 12, 135-141, https://doi.org/10.1134/S181971241802006X.
Nasca, C., Bigio, B., Zelli, D., de Angelis, P., Lau, T., et al. (2017) Role of the Astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress, Neuron, 96, 402-413.e5, https://doi.org/10.1016/j.neuron.2017.09.020.
Beznin, G. V., Pshenichnaya, A. G., Kusov, A. G., and Tsikunov, S. G. (2012) Morphological and functional bases of behavioral deviations in the model of acute vital stress in rats, Med. Akadem. Zhurn., 12, 37-39.
Paxinos, G., and Watson, C. (2007) The Rat Brain in Stereotaxic Coordinates, 6th Edition, Academic Press.
Kopec, A. M., Rivera, P. D., Lacagnina, M. J., Hanamsagar, R., and Bilbo, S. D. (2017) Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue, J. Neurosci. Methods, 280, 64-76, https://doi.org/10.1016/j.jneumeth.2017.02.002.
Harrington, C. R. (1990) Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determinations on fractions from brain tissue, Anal. Biochem., 186, 285-7, https://doi.org/10.1016/0003-2697(90)90081-j.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 227, 680-685, https://doi.org/10.1038/227680a0.
Lei, T., Dong, D., Song, M., Sun, Y., Liu, X., and Zhao, H. (2020) Rislenemdaz treatment in the lateral habenula improves despair-like behavior in mice, Neuropsychopharmacology, 45, 1717-1724, https://doi.org/10.1038/s41386-020-0652-9.
Delawary, M., Tezuka, T., Kiyama, Y., Yokoyama, K., Inoue, T., et al. (2010) NMDAR2B tyrosine phosphorylation regulates anxiety-like behavior and CRF expression in the amygdala, Mol. Brain, 3, 37, https://doi.org/10.1186/1756-6606-3-37.
Rahman, T., Zavitsanou, K., Purves-Tyson, T., Harms, L. R., Meehan, C., et al. (2017) Effects of immune activation during early or late gestation on N-methyl-d-aspartate receptor measures in adult rat offspring, Front. Psychiatry, 8, 77, https://doi.org/10.3389/fpsyt.2017.00077.
Tishkina, A., Stepanichev, M., Kudryashova, I., Freiman, S., Onufriev, M., et al. (2016) Neonatal proinflammatory challenge in male Wistar rats: effects on behavior, synaptic plasticity, and adrenocortical stress response, Behav. Brain Res., 304, 1-10, https://doi.org/10.1016/j.bbr.2016.02.001.
Onufriev, M. V., Freiman, S. V., Peregud, D. I., Kudryashova, I. V., Tishkina, A. O., et al. (2017) Neonatal proinflammatory stress induces accumulation of corticosterone and interleukin-6 in the hippocampus of juvenile rats: potential mechanism of synaptic plasticity impairments, Biochemistry (Moscow), 82, 275-281, https://doi.org/10.1134/S0006297917030051.
Postnikova, T. Y., Griflyuk, A. V., Ergina, J. L., Zubareva, O. E., and Zaitsev, A. V. (2020) Administration of bacterial lipopolysaccharide during early postnatal ontogenesis induces transient impairment of long-term synaptic plasticity associated with behavioral abnormalities in young rats, Pharmaceuticals (Basel), 13, https://doi.org/10.3390/ph13030048.
Onufriev, M. V., Uzakov, S. S., Freiman, S. V., Stepanichev, M. Y., Moiseeva, Y. V., et al. (2018) The Dorsal and ventral hippocampus have different reactivities to proinflammatory stress: corticosterone levels, cytokine expression, and synaptic plasticity, Neurosci. Behav. Physiol., 48, 1024-1031, https://doi.org/10.1007/s11055-018-0665-6.
Benmhammed, H., El Hayek, S., Nassiri, A., Bousalham, R., Mesfioui, A., et al. (2019) Effects of lipopolysaccharide administration and maternal deprivation on anxiety and depressive symptoms in male and female Wistar rats: neurobehavioral and biochemical assessments, Behav. Brain Res., 362, 46-55, https://doi.org/10.1016/j.bbr.2019.01.005.
Sulakhiya, K., Keshavlal, G. P., Bezbaruah, B. B., Dwivedi, S., Gurjar, S. S., et al. (2016) Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin, Neurosci. Lett., 611, 106-111, https://doi.org/10.1016/j.neulet.2015.11.031.
Walker, F. R., March, J., and Hodgson, D. M. (2004) Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat, Behav. Brain Res., 154, 63-69, https://doi.org/10.1016/j.bbr.2004.01.019.
Spencer, S. J., Heida, J. G., and Pittman, Q. J. (2005) Early life immune challenge – effects on behavioural indices of adult rat fear and anxiety, Behav. Brain Res., 164, 231-238, https://doi.org/10.1016/j.bbr.2005.06.032.
Custódio, C. S., Mello, B. S. F., Filho, A. J. M. C., de Carvalho Lima, C. N., Cordeiro, R. C., et al. (2018) Neonatal immune challenge with lipopolysaccharide triggers long-lasting sex- and age-related behavioral and immune/neurotrophic alterations in mice: relevance to autism spectrum disorders, Mol. Neurobiol., 55, 3775-3788, https://doi.org/10.1007/s12035-017-0616-1.
Fan, L.-W., Tien, L.-T., Mitchell, H. J., Rhodes, P. G., and Cai, Z. (2008) Alpha-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide, Eur. J. Neurosci., 27, 1475-84, https://doi.org/10.1111/j.1460-9568.2008.06121.x.
Zoladz, P. R., and Diamond, D. M. (2016) Predator-based psychosocial stress animal model of PTSD: preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis, Exp. Neurol., 284, 211-219, https://doi.org/10.1016/j.expneurol.2016.06.003.
Zoladz, P. R., Park, C. R., Fleshner, M., and Diamond, D. M. (2015) Psychosocial predator-based animal model of PTSD produces physiological and behavioral sequelae and a traumatic memory four months following stress onset, Physiol. Behav., 147, 183-192, https://doi.org/10.1016/j.physbeh.2015.04.032.
Auxéméry, Y. (2012) Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context, Encephale, 38, 373-380, https://doi.org/10.1016/j.encep.2011.12.003.
Ding, X.-F., Li, Y.-H., Chen, J.-X., Sun, L.-J., Jiao, H.-Y., et al. (2017) Involvement of the glutamate/glutamine cycle and glutamate transporter GLT-1 in antidepressant-like effects of Xiao Yao san on chronically stressed mice, BMC Complement. Altern. Med., 17, 326, https://doi.org/10.1186/s12906-017-1830-0.
Shanks, N., Larocque, S., and Meaney, M. J. (1995) Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress, J. Neurosci., 15, 376-384.
Zoladz, P. R., Fleshner, M., and Diamond, D. M. (2012) Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities, Psychoneuroendocrinology, 37, 1531-1545, https://doi.org/10.1016/j.psyneuen.2012.02.007.
Olff, M., Güzelcan, Y., de Vries, G.-J., Assies, J., and Gersons, B. P. R. (2006) HPA- and HPT-axis alterations in chronic posttraumatic stress disorder, Psychoneuroendocrinology, 31, 1220-1230, https://doi.org/10.1016/j.psyneuen.2006.09.003.
Acknowledgments
Spectrophotometric studies and visualization of membranes were carried out at the Center for the Shared Use of Scientific Equipment for Physiological, Biochemical, and Molecular Biology Research at the Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences.
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 17-04-02116).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals have been followed. This article does not describe any research involving human subjects.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Nikitina, V.A., Zakharova, M.V., Trofimov, A.N. et al. Neonatal Exposure to Bacterial Lipopolysaccharide Affects Behavior and Expression of Ionotropic Glutamate Receptors in the Hippocampus of Adult Rats after Psychogenic Trauma. Biochemistry Moscow 86, 761–772 (2021). https://doi.org/10.1134/S0006297921060134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921060134