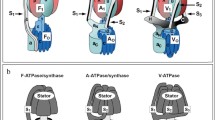
Abstract
Ion-translocating ATPases and ATP synthases (F-, V-, A-type ATPases, and several P-type ATPases and ABC-transporters) catalyze ATP hydrolysis or ATP synthesis coupled with the ion transport across the membrane. F-, V-, and A-ATPases are protein nanomachines that combine transmembrane transport of protons or sodium ions with ATP synthesis/hydrolysis by means of a rotary mechanism. These enzymes are composed of two multisubunit subcomplexes that rotate relative to each other during catalysis. Rotary ATPases phosphorylate/dephosphorylate nucleotides directly, without the generation of phosphorylated protein intermediates. F-type ATPases are found in chloroplasts, mitochondria, most eubacteria, and in few archaea. V-type ATPases are eukaryotic enzymes present in a variety of cellular membranes, including the plasma membrane, vacuoles, late endosomes, and trans-Golgi cisternae. A-type ATPases are found in archaea and some eubacteria. F- and A-ATPases have two main functions: ATP synthesis powered by the proton motive force (pmf) or, in some prokaryotes, sodium-motive force (smf) and generation of the pmf or smf at the expense of ATP hydrolysis. In prokaryotes, both functions may be vitally important, depending on the environment and the presence of other enzymes capable of pmf or smf generation. In eukaryotes, the primary and the most crucial function of F-ATPases is ATP synthesis. Eukaryotic V-ATPases function exclusively as ATP-dependent proton pumps that generate pmf necessary for the transmembrane transport of ions and metabolites and are vitally important for pH regulation. This review describes the diversity of rotary ion-translocating ATPases from different organisms and compares the structural, functional, and regulatory features of these enzymes.





Similar content being viewed by others
References
Beyenbach, K. W. (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation, J. Exp. Biol., 209, 577-589, https://doi.org/10.1242/jeb.02014.
Ihara, K., Abe, T., Sugimura, K. I., and Mukohata, Y. (1992) Halobacterial A-ATP synthase in relation to V-ATPase, J. Exp. Biol., 172, 475-485.
Müller, V., and Grüber, G. (2003) ATP synthases: structure, function and evolution of unique energy converters, Cell. Mol. Life Sci., 60, 474-494, https://doi.org/10.1007/s000180300040.
Grüber, G., and Marshansky, V. (2008) New insights into structure-function relationships between archeal ATP synthase (A1A0) and vacuolar type ATPase (V1V0), BioEssays, 30, 1096-1099, https://doi.org/10.1002/bies.20827.
Kühlbrandt, W. (2019) Structure and mechanisms of F-type ATP synthases, Ann. Rev. Biochem., 88, 515-549, https://doi.org/10.1146/annurev-biochem-013118-110903.
Hilario, E., and Gogarten, J. P. (1998) The prokaryote-to-eukaryote transition reflected in the evolution of the V/F/A-ATPase catalytic and proteolipid subunits, J. Mol. Evol., 46, 703-715, https://doi.org/10.1007/pl00006351.
Mulkidjanian, A. Y., Makarova, K. S., Galperin, M. Y., and Koonin, E. V. (2007) Inventing the dynamo machine: the evolution of the F-type and V-type ATPases, Nat. Rev. Microbiol., 5, 892-899, https://doi.org/10.1038/nrmicro1767.
Gogarten, J. P., and Taiz, L. (1992) Evolution of proton pumping ATPases: rooting the tree of life, Photosynth. Res., 33, 137-146, https://doi.org/10.1007/BF00039176.
Kühlbrandt, W., and Davies, K. M. (2016) Rotary ATPases: a new twist to an ancient machine, Trends Biochem. Sci., 41, 106-116, https://doi.org/10.1016/j.tibs.2015.10.006.
Guo, H., Suzuki, T., and Rubinstein, J. L. (2019) Structure of a bacterial ATP synthase, eLife, 8, https://doi.org/10.7554/eLife.43128.
Gu, J., Zhang, L., Zong, S., Guo, R., Liu, T., et al. (2019) Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1, Science, 364, 1068- 1075, https://doi.org/10.1126/science.aaw4852.
Zhou, L., and Sazanov, L. A. (2019) Structure and conformational plasticity of the intact Thermus thermophilus V/A-type ATPase, Science, 365, https://doi.org/10.1126/science.aaw9144.
Abbas, Y. M., Wu, D., Bueler, S. A., Robinson, C. V., and Rubinstein, J. L. (2020) Structure of V-ATPase from the mammalian brain, Science, 367, 1240-1246, https://doi.org/10.1126/science.aaz2924.
Hahn, A., Vonck, J., Mills, D. J., Meier, T., and Kühlbrandt, W. (2018) Structure, mechanism, and regulation of the chloroplast ATP synthase, Science, 360, https://doi.org/10.1126/science.aat4318.
Murphy, B. J., Klusch, N., Langer, J., Mills, D. J., Yildiz, Ö., and Kühlbrandt, W. (2019) Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-FO coupling, Science, 364, https://doi.org/10.1126/science.aaw9128.
Mühleip, A., McComas, S. E., and Amunts, A. (2019) Structure of a mitochondrial ATP synthase with bound native cardiolipin, eLife, 8, https://doi.org/10.7554/eLife.51179.
Arnold, I., Pfeiffer, K., Neupert, W., Stuart, R. A., and Schägger, H. (1998) Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits, EMBO J., 17, 7170-7178, https://doi.org/10.1093/emboj/17.24.7170.
Guo, H., Bueler, S. A., and Rubinstein, J. L. (2017) Atomic model for the dimeric FO region of mitochondrial ATP synthase, Science, 358, 936-940, https://doi.org/10.1126/science.aao4815.
Eubel, H., Jänsch, L., and Braun, H.-P. (2003) New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II, Plant Physiol., 133, 274-286, https://doi.org/10.1104/pp.103.024620.
Strauss, M., Hofhaus, G., Schröder, R. R., and Kühlbrandt, W. (2008) Dimer ribbons of ATP synthase shape the inner mitochondrial membrane, EMBO J., 27, 1154-1160, https://doi.org/10.1038/emboj.2008.35.
Blum, T. B., Hahn, A., Meier, T., Davies, K. M., and Kühlbrandt, W. (2019) Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows, Proc. Natl. Acad. Sci. USA, 116, 4250-4255, https://doi.org/10.1073/pnas.1816556116.
Paumard, P., Vaillier, J., Coulary, B., Schaeffer, J., Soubannier, V., Mueller, D. M., et al. (2002) The ATP synthase is involved in generating mitochondrial cristae morphology, EMBO J., 21, 221-230, https://doi.org/10.1093/emboj/21.3.221.
Davies, K. M., Anselmi, C., Wittig, I., Faraldo-Gómez, J. D., and Kühlbrandt, W. (2012) Structure of the yeast F1F0-ATP synthase dimer and its role in shaping the mitochondrial cristae, Proc. Natl. Acad. Sci. USA, 109, 13602-13607, https://doi.org/10.1073/pnas.1204593109.
Muench, S. P., Trinick, J., and Harrison, M. A. (2011) Structural divergence of the rotary ATPases, Quart. Rev. Bioph., 44, 311-356, https://doi.org/10.1017/S0033583510000338.
Mazhab-Jafari, M. T., Rohou, A., Schmidt, C., Bueler, S. A., Benlekbir, S., Robinson, C. V., et al. (2016) Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase, Nature, 539, 118-122, https://doi.org/10.1038/nature19828.
Grüber, G., Manimekalai, M. S. S., Mayer, F., and Müller, V. (2014) ATP synthases from archaea: the beauty of a molecular motor, Biochim. Biophys. Acta, 1837, 940-952, https://doi.org/10.1016/j.bbabio.2014.03.004.
Harrison, M. A., and Muench, S. P. (2018) The vacuolar ATPase – a nano-scale motor that drives cell biology, in Membrane Protein Complexes: Structure and Function (Harris, J. R., and Boekema, E. J., eds.) Springer Singapore, Singapore, p. 409-459, https://doi.org/10.1007/978-981-10-7757-9_14.
Vasanthakumar, T., and Rubinstein, J. L. (2020) Structure and roles of V-type ATPases, Trends Biochem. Sci., 45, 295-307, https://doi.org/10.1016/j.tibs.2019.12.007.
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria, Nature, 370, 621-628.
Arai, S., Saijo, S., Suzuki, K., Mizutani, K., Kakinuma, Y., Ishizuka-Katsura, Y., et al. (2013) Rotation mechanism of Enterococcus hirae V1-ATPase based on asymmetric crystal structures, Nature, 493, 703-707, https://doi.org/10.1038/nature11778.
Schäfer, G., Engelhard, M., and Müller, V. (1999) Bioenergetics of the Archaea, Microbiol. Mol. Biol. Rev., 63, 570-620, https://doi.org/10.1128/mmbr.63.3.570-620.1999.
Kumar, A., Manimekalai, M. S. S., Balakrishna, A. M., Jeyakanthan, J., and Grüber, G. (2010) Nucleotide binding states of subunit A of the A-ATP synthase and the implication of P-loop switch in evolution, J. Mol. Biol., 396, 301-320, https://doi.org/10.1016/j.jmb.2009.11.046.
Komoriya, Y., Ariga, T., Iino, R., Imamura, H., Okuno, D., and Noji, H. (2012) Principal role of the arginine finger in rotary catalysis of F1-ATPase, J. Biol. Chem., 287, 15134-15142, https://doi.org/10.1074/jbc.M111.328153.
Malyan, A. N. (2013) Noncatalytic nucleotide binding sites: properties and mechanism of involvement in ATP synthase activity regulation, Biochemistry (Moscow), 78, 1512-1523, https://doi.org/10.1134/S0006297913130099.
Lapashina, A. S., and Feniouk, B. A. (2018) ADP-inhibition of H+-FOF1-ATP synthase, Biochemistry (Moscow), 83, 1141-1160, https://doi.org/10.1134/S0006297918100012.
Suzuki, K., Mizutani, K., Maruyama, S., Shimono, K., Imai, F. L., Muneyuki, E., et al. (2016) Crystal structures of the ATP-binding and ADP-release dwells of the V1 rotary motor, Nat. Commun., 7, 13235, https://doi.org/10.1038/ncomms13235.
Schäfer, I. B., Bailer, S. M., Düser, M. G., Börsch, M., Bernal, R. A., et al. (2006) Crystal structure of the archaeal A1AO ATP synthase subunit B from Methanosarcina mazei Gö1: implications of nucleotide-binding differences in the major A1AO subunits A and B, J. Mol. Biol., 358, 725-740, https://doi.org/10.1016/j.jmb.2006.02.057.
Gogarten, J. P., Kibak, H., Dittrich, P., Taiz, L., Bowman, E. J., Bowman, B. J., et al. (1989) Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes, Proc. Natl. Acad. Sci. USA, 86, 6661-6665, https://doi.org/10.1073/pnas.86.17.6661.
Boyer, P. D. (1997) The ATP synthase – a splendid molecular machine, Annu. Rev. Biochem., 66, 717-749.
Noji, H., Yasuda, R., Yoshida, M., and Kinosita, K. (1997) Direct observation of the rotation of F1-ATPase, Nature, 386, 299-302, https://doi.org/10.1038/386299a0.
Okuno, D., Iino, R., and Noji, H. (2011) Rotation and structure of FOF1-ATP synthase, J. Biochem., 149, 655-664, https://doi.org/10.1093/jb/mvr049.
Junge, W., and Nelson, N. (2015) ATP synthase, Annu. Rev. Biochem., 84, 631-657, https://doi.org/10.1146/annurev-biochem-060614-034124.
Noji, H., Ueno, H., and McMillan, D. G. G. (2017) Catalytic robustness and torque generation of the F1-ATPase, Biophys. Rev., 9, 103-118, https://doi.org/10.1007/s12551-017-0262-x.
Iida, T., Minagawa, Y., Ueno, H., Kawai, F., Murata, T., and Iino, R. (2019) Single-molecule analysis reveals rotational substeps and chemo-mechanical coupling scheme of V-ATPase, J. Biol. Chem., 294, 17017-17030, https://doi.org/10.1074/jbc.RA119.008947.
Furuike, S., Nakano, M., Adachi, K., Noji, H., Kinosita, K., and Yokoyama, K. (2011) Resolving stepping rotation in Thermus thermophilus H(+)-ATPase/synthase with an essentially drag-free probe, Nat. Commun., 2, 233, https://doi.org/10.1038/ncomms1215.
Imamura, H., Takeda, M., Funamoto, S., Shimabukuro, K., Yoshida, M., and Yokoyama, K. (2005) Rotation scheme of V1-motor is different from that of F1-motor, Proc. Natl. Acad. Sci. USA, 102, 17929-17933.
Hirata, T., Iwamoto-Kihara, A., Sun-Wada, G.-H., Okajima, T., Wada, Y., and Futai, M. (2003) Subunit rotation of vacuolar-type proton pumping ATPase: relative rotation of the G and C subunits, J. Biol. Chem., 278, 23714-23719, https://doi.org/10.1074/jbc.M302756200.
Noji, H., Bald, D., Yasuda, R., Itoh, H., Yoshida, M., and Kinosita, K. (2001) Purine but not pyrimidine nucleotides support rotation of F(1)-ATPase, J. Biol. Chem., 276, 25480-25486, https://doi.org/10.1074/jbc.M102200200.
Pisa, K. Y., Huber, H., Thomm, M., and Müller, V. (2007) A sodium ion-dependent A1AO ATP synthase from the hyperthermophilic archaeon Pyrococcus furiosus: A1AO ATPase of Pyrococcus furiosus, FEBS J., 274, 3928-3938, https://doi.org/10.1111/j.1742-4658.2007.05925.x.
Yokoyama, K., Akabane, Y., Ishii, N., and Yoshida, M. (1994) Isolation of prokaryotic VOV1-ATPase from a thermophilic eubacterium Thermus thermophilus, J. Biol. Chem., 269, 12248-12253.
Yoshida, M., Sone, N., Hirata, H., and Kagawa, Y. (1975) A highly stable adenosine triphosphatase from a thermophilic bacterium. Purification, properties, and reconstitution, J. Biol. Chem., 250, 7910-7916.
Senior, A. E., Lee, R. S., al-Shawi, M. K., and Weber, J. (1992) Catalytic properties of Escherichia coli F1-ATPase depleted of endogenous nucleotides, Arch. Biochem. Biophys., 297, 340-344.
Iida, T., Hoaki, T., Kamino, K., Inatomi, K., Kamagata, Y., and Maruyama, T. (1996) Vacuolar-type ATPase in a hyperthermophilic archaeum, Thermococcus sp., Biochem. Biophys. Res. Commun., 229, 559-564, https://doi.org/10.1006/bbrc.1996.1843.
Konishi, J., Wakagi, T., Oshima, T., and Yoshida, M. (1987) Purification and properties of the ATPase solubilized from membranes of an acidothermophilic archaebacterium, Sulfolobus acidocaldarius, J. Biochem., 102, 1379-1387, https://doi.org/10.1093/oxfordjournals.jbchem.a122184.
Pedersen, P. L. (1976) ATP-dependent reactions catalyzed by inner membrane vesicles of rat liver mitochondria. Kinetics, substrate specificity, and bicarbonate sensitivity, J. Biol. Chem., 251, 934-940.
Vambutas, V. K., and Racker, E. (1965) Partial resolution of the enzymes catalyzing photophosphorylation. I. Stimulation of photophosphorylation by a preparation of a latent, Ca++-dependent adenosine triphosphatase from chloroplasts, J. Biol. Chem., 240, 2660-2667.
Struve, I., and Lüttge, U. (1987) Characteristics of MgATP2–-dependent electrogenic proton transport in tonoplast vesicles of the facultative crassulacean-acid-metabolism plant Mesembryanthemum crystallinum L., Planta, 170, 111-120, https://doi.org/10.1007/BF00392387.
Pacheco, G., Lippo de Bécemberg, I., Gonzalez de Alfonzo, R., and Alfonzo, M. J. (1996) Biochemical characterization of a V-ATPase of tracheal smooth muscle plasma membrane fraction, Biochim. Biophys. Acta, 1282, 182-192, https://doi.org/10.1016/0005-2736(96)00038-7.
Perlin, D. S., Latchney, L. R., Wise, J. G., and Senior, A. E. (1984) Specificity of the proton adenosine triphosphatase of Escherichia coli for adenine, guanine, and inosine nucleotides in catalysts and binding, Biochemistry, 23, 4998-5003, https://doi.org/10.1021/bi00316a026.
Suzuki, T., Wakabayashi, C., Tanaka, K., Feniouk, B. A., and Yoshida, M. (2011) Modulation of nucleotide specificity of thermophilic FOF1-ATP synthase by epsilon-subunit, J. Biol. Chem., 286, 16807-16813, https://doi.org/10.1074/jbc.M110.209965.
D’Auzac, J. (1977) ATPase membranaire de vacuoles lysosomales: les lutoides du latex d’Hevea brasiliensis, Phytochemistry, 16, 1881-1885, https://doi.org/10.1016/0031-9422(77)80088-5.
Gräf, R., Harvey, W. R., and Wieczorek, H. (1996) Purification and properties of a Cytosolic V1-ATPase, J. Biol. Chem., 271, 20908-20913, https://doi.org/10.1074/jbc.271.34.20908.
Mayer, F., Lim, J. K., Langer, J. D., Kang, S. G., and Mueller, V. (2015) Na+ transport by the A(1)A(O)-ATP synthase purified from Thermococcus onnurineus and reconstituted into liposomes, J. Biol. Chem., 290, 6994-7002, https://doi.org/10.1074/jbc.M114.616862.
Valiyaveetil, F. I., and Fillingame, R. H. (1997) On the role of Arg-210 and Glu-219 of subunit a in proton translocation by the Escherichia coli FOF1-ATP synthase, J. Biol. Chem., 272, 32635-32641, https://doi.org/10.1074/jbc.272.51.32635.
Glagolev, A. N., and Skulachev, V. P. (1978) The proton pump is a molecular engine of motile bacteria, Nature, 272, 280-282, https://doi.org/10.1038/272280a0.
Junge, W., Lill, H., and Engelbrecht, S. (1997) ATP synthase: an electrochemical transducer with rotatory mechanics, Trends Biochem. Sci., 22, 420-423, https://doi.org/10.1016/s0968-0004(97)01129-8.
Vik, S. B., and Antonio, B. J. (1994) A mechanism of proton translocation by F1FO ATP synthases suggested by double mutants of the a subunit, J. Biol. Chem., 269, 30364-30369.
Srivastava, A. P., Luo, M., Zhou, W., Symersky, J., Bai, D., Chambers, M. G., et al. (2018) High-resolution cryo-EM analysis of the yeast ATP synthase in a lipid membrane, Science, 360, https://doi.org/10.1126/science.aas9699.
Murata, T., Yamato, I., Kakinuma, Y., Leslie, A. G. W., and Walker, J. E. (2005) Structure of the rotor of the V-Type Na+-ATPase from Enterococcus hirae, Science, 308, 654-659, https://doi.org/10.1126/science.1110064.
Skulachev, V. P. (1984) Membrane bioenergetics – should we build the bridge across the river or alongside of it? Trends Biochem. Sci., 9, 182-185, https://doi.org/10.1016/0968-0004(84)90134-8.
Skulachev, V. P. (1985) Membrane-linked energy transductions. Bioenergetic functions of sodium: H+ is not unique as a coupling ion, Eur. J. Biochem., 151, 199-208, https://doi.org/10.1111/j.1432-1033.1985.tb09088.x.
Dimroth, P., and Cook, G. M. (2004) Bacterial Na+- or H+-coupled ATP synthases operating at low electrochemical potential, Adv. Microb. Physiol., 49, 175-218.
Mulkidjanian, A. Y., Dibrov, P., and Galperin, M. Y. (2008) The past and present of sodium energetics: may the sodium-motive force be with you, Biochim. Biophys. Acta, 1777, 985-992, https://doi.org/10.1016/j.bbabio.2008.04.028.
Mulkidjanian, A. Y., Galperin, M. Y., Makarova, K. S., Wolf, Y. I., and Koonin, E. V. (2008) Evolutionary primacy of sodium bioenergetics, Biol. Direct., 3, 13, https://doi.org/10.1186/1745-6150-3-13.
Poehlein, A., Schmidt, S., Kaster, A.-K., Goenrich, M., Vollmers, J., et al. (2012) An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis, PLoS One, 7, e33439, https://doi.org/10.1371/journal.pone.0033439.
Dibrova, D. V., Galperin, M. Y., and Mulkidjanian, A. Y. (2010) Characterization of the N-ATPase, a distinct, laterally transferred Na+-translocating form of the bacterial F-type membrane ATPase, Bioinformatics, 26, 1473-1476, https://doi.org/10.1093/bioinformatics/btq234.
Schulz, S., WiIkes, M., Mills, D. J., Kuhlbrandt, W., and Meier, T. (2017) Molecular archifecture of the N-type ATPase rotor ring from Barkholderia pseudomallei, EMBO Rep., 18, 526-535, https://doi.org/10.15252/embr.201643374.
Laubinger, W., and Dimroth, P. (1989) The sodium ion translocating adenosine triphosphatase of Propionigenium modestum pumps protons at low sodium ion concentrations, Biochemistry, 28, 7194-7198, https://doi.org/10.1021/bi00444a010.
Neumann, S., Matthey, U., and Kaim, G. (1998) Purification and properties of the F1Fo ATPase of Ilyobacter tartaricus, a sodium ion pump, J. Bacteriol., 180, 3312-3316, https://doi.org/10.1128/JB.180.13.3312-3316.1998.
McMillan, D. G. G., Ferguson, S. A., Dey, D., Schröder, K., Aung, H. L., Carbone, V., et al. (2011) A1Ao-ATP synthase of Methanobrevibacter ruminantium couples sodium ions for ATP synthesis under physiological conditions, J. Biol. Chem., 286, 39882-39892, https://doi.org/10.1074/jbc.M111.281675.
Murata, T., Yamato, I., Kakinuma, Y., Shirouzu, M., Walker, J. E., Yokoyama, S., et al. (2008) Ion binding and selectivity of the rotor ring of the Na+-transporting V-ATPase, Proc. Natl. Acad. Sci. USA, 105, 8607-8611, https://doi.org/10.1073/pnas.0800992105.
Meier, T., Krah, A., Bond, P. J., Pogoryelov, D., Diederichs, K., and Faraldo-Gómez, J. D. (2009) Complete ion-coordination structure in the rotor ring of Na+-dependent F-ATP synthases, J. Mol. Biol., 391, 498-507, https://doi.org/10.1016/j.jmb.2009.05.082.
Krah, A., Pogoryelov, D., Langer, J. D., Bond, P. J., Meier, T., and Faraldo-Gómez, J. D. (2010) Structural and energetic basis for H+ versus Na+ binding selectivity in ATP synthase FO rotors, Biochim. Biophys. Acta, 1797, 763-772, https://doi.org/10.1016/j.bbabio.2010.04.014.
Leone, V., Pogoryelov, D., Meier, T., and Faraldo-Gómez, J. D. (2015) On the principle of ion selectivity in Na+/H+-coupled membrane proteins: experimental and theoretical studies of an ATP synthase rotor, Proc. Natl. Acad. Sci. USA, 112, 1057-1066, https://doi.org/10.1073/pnas.1421202112.
Schlegel, K., Leone, V., Faraldo-Gómez, J. D., and Müller, V. (2012) Promiscuous archaeal ATP synthase concurrently coupled to Na+ and H+ translocation, Proc. Natl. Acad. Sci. USA, 109, 947-952, https://doi.org/10.1073/pnas.1115796109.
Watt, I. N., Montgomery, M. G., Runswick, M. J., Leslie, A. G. W., and Walker, J. E. (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria, Proc. Natl. Acad. Sci. USA, 107, 16823-16827, https://doi.org/10.1073/pnas.1011099107.
Ruppert, C., Kavermann, H., Wimmers, S., Schmid, R., Kellermann, J., Lottspeich, F., et al. (1999) The proteolipid of the A1AO ATP synthase from Methanococcus jannaschii has six predicted transmembrane helices but only two proton-translocating carboxyl groups, J. Biol. Chem., 274, 25281-25284, https://doi.org/10.1074/jbc.274.36.25281.
Wilms, R., Freiberg, C., Wegerle, E., Meier, I., Mayer, F., and Müller, V. (1996) Subunit structure and organization of the genes of the A1AO ATPase from the Archaeon Methanosarcina mazei Gö1, J. Biol. Chem., 271, 18843-18852, https://doi.org/10.1074/jbc.271.31.18843.
Steinert, K., Wagner, V., Kroth-Pancic, P. G., and Bickel-Sandkötter, S. (1997) Characterization and subunit structure of the ATP synthase of the halophilic archaeon Haloferax volcanii and organization of the ATP synthase genes, J. Biol. Chem., 272, 6261-6269, https://doi.org/10.1074/jbc.272.10.6261.
Kibak, H., Taiz, L., Starke, T., Bernasconi, P., and Gogarten, J. P. (1992) Evolution of structure and function of V-ATPases, J. Bioenerg. Biomembr., 24, 415-424, https://doi.org/10.1007/BF00762534.
Ihara, K., Watanabe, S., Sugimura, K.-I., Katagiri, I., and Mukohata, Y. (1997) Identification of proteolipid from an extremely halophilic archaeon Halobacterium salinarumas an N, N′-dicyclohexyl-carbodiimide binding subunit of ATP synthase, Arch. Biochem. Biophys., 341, 267-272, https://doi.org/10.1006/abbi.1997.9972.
Vonck, J., Pisa, K. Y., Morgner, N., Brutschy, B., and Müller, V. (2009) Three-dimensional structure of A1AO ATP synthase from the hyperthermophilic archaeon Pyrococcus furiosus by electron microscopy, J. Biol. Chem., 284, 10110-10119, https://doi.org/10.1074/jbc.M808498200.
Mayer, F., Leone, V., Langer, J. D., Faraldo-Gómez, J. D., and Müller, V. (2012) A c subunit with four transmembrane helices and one ion Na+-binding site in an archaeal ATP synthase: implications for c ring function and structure, J. Biol. Chem., 287, 39327-39337, https://doi.org/10.1074/jbc.M112.411223.
Slesarev, A. I., Mezhevaya, K. V., Makarova, K. S., Polushin, N. N., Shcherbinina, O. V., et al. (2002) The complete genome of hyperthermophile Methanopyrus kandleri AV19 and monophyly of archaeal methanogens, Proc. Natl. Acad. Sci. USA, 99, 4644-4649, https://doi.org/10.1073/pnas.032671499.
Müller, V., Aufurth, S., and Rahlfs, S. (2001) The Na+ cycle in Acetobacterium woodii: identification and characterization of a Na+ translocating F1F0-ATPase with a mixed oligomer of 8 and 16 kDa proteolipids, Biochim. Biophys. Acta Bioenergetics, 1505, 108-120, https://doi.org/10.1016/S0005-2728(00)00281-4.
Matthies, D., Zhou, W., Klyszejko, A. L., Anselmi, C., Yildiz, Ö., Brandt, K., et al. (2014) High-resolution structure and mechanism of an F/V-hybrid rotor ring in a Na+-coupled ATP synthase, Nat. Commun., 5, 5286, https://doi.org/10.1038/ncomms6286.
Pogoryelov, D., Klyszejko, A. L., Krasnoselska, G. O., Heller, E.-M., Leone, V., et al. (2012) Engineering rotor ring stoichiometries in the ATP synthase, Proc. Natl. Acad. Sci. USA, 109, 1599-1608, https://doi.org/10.1073/pnas.1120027109.
Preiss, L., Klyszejko, A. L., Hicks, D. B., Liu, J., Fackelmayer, O. J., et al. (2013) The c-ring stoichiometry of ATP synthase is adapted to cell physiological requirements of alkaliphilic Bacillus pseudofirmus OF4, Proc. Natl. Acad. Sci. USA, 110, 7874-7879, https://doi.org/10.1073/pnas.1303333110.
Veech, R. L., King, M. T., Pawlosky, R., Bradshaw, P. C., and Curtis, W. (2019) Relationship between inorganic ion distribution, resting membrane potential, and the ΔG’ of ATP hydrolysis: a new paradigm, FASEB J., 33, 13126-13130, https://doi.org/10.1096/fj.201901942R.
Hüttemann, M., Lee, I., Pecinova, A., Pecina, P., Przyklenk, K., and Doan, J. W. (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease, J. Bioener. Biomembr., 40, 445-456, https://doi.org/10.1007/s10863-008-9169-3.
Hisabori, T., Konno, H., Ichimura, H., Strotmann, H., and Bald, D. (2002) Molecular devices of chloroplast F1-ATP synthase for the regulation, Biochim. Biophys. Acta Bioenergetics, 1555, 140-146, https://doi.org/10.1016/S0005-2728(02)00269-4.
Galperin, M. Y., Makarova, K. S., Wolf, Y. I., and Koonin, E. V. (2015) Expanded microbial genome coverage and improved protein family annotation in the COG database, Nucleic Acids Res., 43, 261-269, https://doi.org/10.1093/nar/gku1223.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiol. Rev., 94, 909-950, https://doi.org/10.1152/physrev.00026.2013.
Giorgio, V., von Stockum, S., Antoniel, M., Fabbro, A., Fogolari, F., Forte, M., et al. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore, Proc. Natl. Acad. Sci. USA, 110, 5887-5892, https://doi.org/10.1073/pnas.1217823110.
Carroll, J., He, J., Ding, S., Fearnley, I. M., and Walker, J. E. (2019) Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase, Proc. Natl. Acad. Sci. USA, 116, 12816-12821, https://doi.org/10.1073/pnas.1904005116.
Hirata, T., Nakamura, N., Omote, H., Wada, Y., and Futai, M. (2000) Regulation and reversibility of vacuolar H+-ATPase, J. Biol. Chem., 275, 386-389, https://doi.org/10.1074/jbc.275.1.386.
Forgac, M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology, Nat. Rev. Mol. Cell Biol., 8, 917-929, https://doi.org/10.1038/nrm2272.
McGuire, C., Stransky, L., Cotter, K., and Forgac, M. (2017) Regulation of V-ATPase activity, Front. Biosci., 22, 609-622, https://doi.org/10.2741/4506.
Huang, C., and Chang, A. (2011) pH-dependent cargo sorting from the Golgi, J. Biol. Chem., 286, 10058-10065, https://doi.org/10.1074/jbc.M110.197889.
Kozik, P., Hodson, N. A., Sahlender, D. A., Simecek, N., Soromani, C., Wu, J., et al. (2013) A human genome-wide screen for regulators of clathrin-coated vesicle formation reveals an unexpected role for the V-ATPase, Nat. Cell Biol., 15, 50-60, https://doi.org/10.1038/ncb2652.
Futai, M., Sun-Wada, G.-H., Wada, Y., Matsumoto, N., and Nakanishi-Matsui, M. (2019) Vacuolar-type ATPase: a proton pump to lysosomal trafficking, Proc. Japan Acad. Series B Physic. Biol. Sci., 95, 261-277, https://doi.org/10.2183/pjab.95.018.
Finberg, K. E., Wagner, C. A., Bailey, M. A., Paunescu, T. G., Breton, S., Brown, D., et al. (2005) The B1-subunit of the H+ ATPase is required for maximal urinary acidification, Proc. Natl. Acad. Sci. USA, 102, 13616-13621, https://doi.org/10.1073/pnas.0506769102.
Cotter, K., Stransky, L., McGuire, C., and Forgac, M. (2015) Recent insights into the structure, regulation, and function of the V-ATPases, Trends Biochem. Sci., 40, 611-622, https://doi.org/10.1016/j.tibs.2015.08.005.
Almeida, D. M., Oliveira, M. M., and Saibo, N. J. M. (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants, Genet. Mol. Biol., 40, 326-345, https://doi.org/10.1590/1678-4685-GMB-2016-0106.
Zoncu, R., Bar-Peled, L., Efeyan, A., Wang, S., Sancak, Y., and Sabatini, D. M. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase, Science, 334, 678-683, https://doi.org/10.1126/science.1207056.
Feniouk, B. A., and Yoshida, M. (2008) Regulatory mechanisms of proton-translocating FOF1-ATP synthase, Results Problems Cell Differ., 45, 279-308, https://doi.org/10.1007/400_2007_043.
Feniouk, B. A., Suzuki, T., and Yoshida, M. (2006) The role of subunit epsilon in the catalysis and regulation of FOF1-ATP synthase, Biochim. Biophys. Acta, 1757, 326-338, https://doi.org/10.1016/j.bbabio.2006.03.022.
Gledhill, J. R., Montgomery, M. G., Leslie, A. G. W., and Walker, J. E. (2007) How the regulatory protein, IF1, inhibits F1-ATPase from bovine mitochondria, Proc. Natl. Acad. Sci. USA, 104, 15671-15676, https://doi.org/10.1073/pnas.0707326104.
Morales-Ríos, E., de la Rosa-Morales, F., Mendoza-Hernández, G., Rodríguez-Zavala, J. S., Celis, H., et al. (2010) A novel 11-kDa inhibitory subunit in the F1FO ATP synthase of Paracoccus denitrificans and related alpha-proteobacteria, FASEB J., 24, 599-608, https://doi.org/10.1096/fj.09-137356.
Yokoyama, K., Muneyuki, E., Amano, T., Mizutani, S., Yoshida, M., Ishida, M., et al. (1998) V-ATPase of Thermus thermophilus is inactivated during ATP hydrolysis but can synthesize ATP, J. Biol. Chem., 273, 20504-20510, https://doi.org/10.1074/jbc.273.32.20504.
Nakano, M., Imamura, H., Toei, M., Tamakoshi, M., Yoshida, M., and Yokoyama, K. (2008) ATP hydrolysis and synthesis of a rotary motor V-ATPase from Thermus thermophilus, J. Biol. Chem., 283, 20789-20796, https://doi.org/10.1074/jbc.M801276200.
Singh, D., and Grüber, G. (2018) Crystallographic and enzymatic insights into the mechanisms of Mg-ADP inhibition in the A1 complex of the A1AO ATP synthase, J. Struct. Biol., 201, 26-35, https://doi.org/10.1016/j.jsb.2017.10.008.
Kishikawa, J.-I., Nakanishi, A., Furuike, S., Tamakoshi, M., and Yokoyama, K. (2014) Molecular basis of ADP inhibition of vacuolar (V)-type ATPase/synthase, J. Biol. Chem., 289, 403-412, https://doi.org/10.1074/jbc.M113.523498.
Lapashina, A. S., Prikhodko, A. S., Shugaeva, T. E., and Feniouk, B. A. (2019) Residue 249 in subunit beta regulates ADP inhibition and its phosphate modulation in Escherichia coli ATP synthase, Biochim. Biophys. Acta Bioenerg., 1860, 181-188, https://doi.org/10.1016/j.bbabio.2018.12.003.
Lapashina, A. S., and Feniouk, B. A. (2019) Mutation Q259L in subunit beta in Bacillus subtilis ATP synthase attenuates ADP-inhibition and decreases fitness in mixed cultures, Biochem. Biophys. Res. Commun., 509, 102-107, https://doi.org/10.1016/j.bbrc.2018.12.075.
David, P., and Baron, R. (1994) The catalytic cycle of the vacuolar H+-ATPase. Comparison of proton transport in kidney- and osteoclast-derived vesicles, J. Biol. Chem., 269, 30158- 30163.
Moriyama, Y., and Nelson, N. (1987) Nucleotide binding sites and chemical modification of the chromaffin granule proton ATPase, J. Biol. Chem., 262, 14723-14729.
Webster, L. C., Pérez-Castiñeira, J. R., Atkins, G. L., and Apps, D. K. (1995) Allosteric regulation of proton translocation by a vacuolar adenosinetriphosphatase, Eur. J. Biochem., 232, 586-595, https://doi.org/10.1111/j.1432-1033.1995.586zz.x.
Vasilyeva, E., and Forgac, M. (1998) Interaction of the clathrin-coated vesicle V-ATPase with ADP and sodium azide, J. Biol. Chem., 273, 23823-23829, https://doi.org/10.1074/jbc.273.37.23823.
Kishikawa, J.-I., Seino, A., Nakanishi, A., Tirtom, N. E., Noji, H., Yokoyama, K., et al. (2014) F-subunit reinforces torque generation in V-ATPase, Eur. Biophys. J., 43, 415-422, https://doi.org/10.1007/s00249-014-0973-x.
Singh, D., Sielaff, H., Sundararaman, L., Bhushan, S., and Grüber, G. (2016) The stimulating role of subunit F in ATPase activity inside the A1-complex of the Methanosarcina mazei Gö1 A1AO ATP synthase, Biochim. Biophys. Acta, 1857, 177-187, https://doi.org/10.1016/j.bbabio.2015.12.003.
Singh, D., Sielaff, H., Börsch, M., and Grüber, G. (2017) Conformational dynamics of the rotary subunit F in the A3B3DF complex of Methanosarcina mazei Gö1 A-ATP synthase monitored by single-molecule FRET, FEBS Lett., 591, 854-862, https://doi.org/10.1002/1873-3468.12605.
Saijo, S., Arai, S., Hossain, K. M. M., Yamato, I., Suzuki, K., Kakinuma, Y., et al. (2011) Crystal structure of the central axis DF complex of the prokaryotic V-ATPase, Proc. Natl. Acad. Sci. USA, 108, 19955-19960, https://doi.org/10.1073/pnas.1108810108.
Akanuma, G., Tagana, T., Sawada, M., Suzuki, S., Shimada, T., Tanaka, K., et al. (2019) C-terminal regulatory domain of the ε subunit of FOF1 ATP synthase enhances the ATP-dependent H+ pumping that is involved in the maintenance of cellular membrane potential in Bacillus subtilis, Microbiol. Open, 8, e00815, https://doi.org/10.1002/mbo3.815.
Kishikawa, J.-I., Ibuki, T., Nakamura, S., Nakanishi, A., Minamino, T., Miyata, T., et al. (2013) Common evolutionary origin for the rotor domain of rotary ATPases and flagellar protein export apparatus, PLoS One, 8, e64695, https://doi.org/10.1371/journal.pone.0064695.
Balakrishna, A. M., Basak, S., Manimekalai, M. S. S., and Grüber, G. (2015) Crystal structure of subunits D and F in complex gives insight into energy transmission of the eukaryotic V-ATPase from Saccharomyces cerevisiae, J. Biol. Chem., 290, 3183-3186, https://doi.org/10.1074/jbc.M114.622688.
Kane, P. M. (2012) Targeting reversible disassembly as a mechanism of controlling V-ATPase activity, Curr. Protein Peptide Sci., 13, 117-123, https://doi.org/10.2174/138920312800493142.
Tabke, K., Albertmelcher, A., Vitavska, O., Huss, M., Schmitz, H.-P., and Wieczorek, H. (2014) Reversible disassembly of the yeast V-ATPase revisited under in vivo conditions, Biochem. J., 462, 185-197, https://doi.org/10.1042/BJ20131293.
Beltrán, C., and Nelson, N. (1992) The membrane sector of vacuolar H(+)-ATPase by itself is impermeable to protons, Acta Physiol. Scandinavica Suppl., 607, 41-47.
Zhang, J., Myers, M., and Forgac, M. (1992) Characterization of the VO domain of the coated vesicle (H+)-ATPase, J. Biol. Chem., 267, 9773-9778.
Qi, J., and Forgac, M. (2008) Function and subunit interactions of the N-terminal domain of subunit a (Vph1p) of the yeast V-ATPase, J. Biol. Chem., 283, 19274-19282, https://doi.org/10.1074/jbc.M802442200.
Couoh-Cardel, S., Milgrom, E., and Wilkens, S. (2015) Affinity purification and structural features of the yeast vacuolar ATPase VO membrane sector, J. Biol. Chem., 290, 27959-27971, https://doi.org/10.1074/jbc.M115.662494.
Hayek, S. R., Rane, H. S., and Parra, K. J. (2019) Reciprocal regulation of V-ATPase and glycolytic pathway elements in health and disease, Front. Physiol., 10, 127, https://doi.org/10.3389/fphys.2019.00127.
Lu, M., Ammar, D., Ives, H., Albrecht, F., and Gluck, S. L. (2007) Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump, J. Biol. Chem., 282, 24495-24503, https://doi.org/10.1074/jbc.M702598200.
Chan, C.-Y., and Parra, K. J. (2014) Yeast phosphofructokinase-1 subunit Pfk2p is necessary for pH homeostasis and glucose-dependent vacuolar ATPase reassembly, J. Biol. Chem., 289, 19448-19457, https://doi.org/10.1074/jbc.M114.569855.
Feng, Y., and Forgac, M. (1992) A novel mechanism for regulation of vacuolar acidification, J. Biol. Chem., 267, 19769-19772.
Feng, Y., and Forgac, M. (1994) Inhibition of vacuolar H+-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A, J. Biol.Chem., 269, 13224-13230.
Forgac, M. (1999) The vacuolar H+-ATPase of clathrin-coated vesicles is reversibly inhibited by S-nitrosoglutathione, J. Biol. Chem., 274, 1301-1305, https://doi.org/10.1074/jbc.274.3.1301.
Dschida, W. J., and Bowman, B. J. (1995) The vacuolar ATPase: sulfite stabilization and the mechanism of nitrate inactivation, J. Biol. Chem., 270, 1557-1563, https://doi.org/10.1074/jbc.270.4.1557.
Liu, Q., Leng, X. H., Newman, P. R., Vasilyeva, E., Kane, P. M., and Forgac, M. (1997) Site-directed mutagenesis of the yeast V-ATPase A subunit, J. Biol. Chem., 272, 11750-11756, https://doi.org/10.1074/jbc.272.18.11750.
Oluwatosin, Y. E., and Kane, P. M. (1997) Mutations in the CYS4 gene provide evidence for regulation of the yeast vacuolar H+-ATPase by oxidation and reduction in vivo, J. Biol. Chem., 272, 28149-28157, https://doi.org/10.1074/jbc.272.44.28149.
Hager, A., and Lanz, C. (1989) Essential sulfhydryl groups in the catalytic center of the tonoplast H+-ATPase from coleoptiles of Zea mays L. as demonstrated by the biotin-streptavidin-peroxidase system, Planta, 180, 116-212, https://doi.org/10.1007/BF02411417.
Seidel, T., Scholl, S., Krebs, M., Rienmüller, F., Marten, I., Hedrich, R., et al. (2012) Regulation of the V-type ATPase by redox modulation, Biochem. J., 448, 243-251, https://doi.org/10.1042/BJ20120976.
Hards, K., and Cook, G. M. (2018) Targeting bacterial energetics to produce new antimicrobials, Drug Resist. Updates: Rev.Comm. Antimicrob. Anticancer Chemother., 36, 1-12, https://doi.org/10.1016/j.drup.2017.11.001.
Acknowledgements
The authors are grateful to Vladimir P. Skulachev for the unique and inspiring atmosphere at the Belozersky Institute of Physico-Chemical Biology of Lomonosov Moscow State University (MSU). The authors also highly appreciate his effort in founding the MSU Faculty of Bioengineering and Bioinformatics, without which this work would never have been written.
Funding
This work was supported by the Russian Science Foundation (project no. 20-14-00268).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain a description of studies with the involvement of humans or animal subjects.
Rights and permissions
About this article
Cite this article
Zubareva, V.M., Lapashina, A.S., Shugaeva, T.E. et al. Rotary Ion-Translocating ATPases/ATP Synthases: Diversity, Similarities, and Differences. Biochemistry Moscow 85, 1613–1630 (2020). https://doi.org/10.1134/S0006297920120135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920120135