Abstract
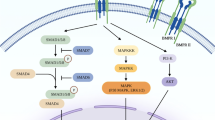
Hyaline cartilage is a nonvascular connective tissue covering the joint surface. It consists mostly of the extracellular matrix proteins and a small number of highly differentiated chondrocytes. At present, various techniques for repairing joint surfaces damage, for example, the use of modified cell cultures and biodegradable scaffolds, are under investigation. Molecular mechanisms of cartilage tissue proliferation have been also actively studied in recent years. TGFβ3, which plays a critical role in the proliferation of normal cartilage tissue, is one of the most important protein among cytokines and growth factors affecting chondrogenesis. By interacting directly with receptors on the cell membrane surface, TGFβ3 triggers a cascade of molecular interactions involving transcription factor Sox9. In this review, we describe the effects of TGFβ3 on the receptor complex activation and subsequent intracellular trafficking of Smad proteins and analyze the relation between these processes and upregulation of expression of major extracellular matrix genes, such as col2a1 and acan.

Similar content being viewed by others
Abbreviations
- BMP:
-
bone morphogenetic protein
- CTEC:
-
cell-based tissue engineered construct
- FSH:
-
follicle-stimulating hormone
- GDF:
-
growth differentiation factor
- MIF:
-
Müllerian inhibiting factor
- MMSC:
-
mesenchymal multipotent stromal cell
- SARA:
-
Smad anchor for receptor activation
- SBE:
-
Smad-binding element
- Sp1:
-
special protein
- SUM:
-
small ubiquitin-like modifier
- TGFβ:
-
transforming growth factor
REFERENCES
Bozhokin, M. S., Bozhkova, S. A., and Netyl’ko G. I. (2016) Opportunities of contemporary cell technologies for recovery of damaged articular cartilage (analytical review) [in Russian], Travmatol. Ortoped. Ross., 22, 122-134, doi: 10.21823/2311-2905-2016-22-3-122-134.
Sophia Fox, A. J., Bedi, A., and Rodeo, S. A. (2009) The basic science of articular cartilage: structure, composition, and function, Sports Health, 1, 461-468, doi: 10.1177/1941738109350438.
Bozhokin, M. S., Bozhkova, S. A., Netyl’ko, G. I., Rumakin, V. P., Nakonechnyy, D. G., and Chepurnenko, M. N. (2017) Morphofunctional characteristics of chondroregenerative process in experimental local superficial defect of articular cartilage [in Russian], Mezhdunarod. Zhurn. Prikl. Fundamental. Issled., 8, 302-306.
Maglio, M., Brogini, S., Pagani, S., Giavaresi, G., and Tschon, M. (2019) Current trends in the evaluation of osteochondral lesion treatments: histology, histomorphometry, and biomechanics in preclinical models, Bio. Med. Res. Intern., 2019, 4040236, doi: 10.1155/2019/4040236.
Kurtz, S., Ong, K., Lau, E., Mowat, F., and Halpern, M. (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030, J. Bone Joint Surg. Am., 89, 780-785, doi: 10.2106/JBJS.F.00222.
Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., and Peterson, L. (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation, N. Engl. J. Med., 6, 889-895, doi: 10.1056/NEJM199410063311401.
Xiang, Y., Bunpetch, V., Zhou, W., and Ouyang, H. (2019) Optimization strategies for ACI: a step-chronicle review, J. Orthop. Translat., 17, 3-14, doi: 10.1016/j.jot.2018.12.005.
Elmallah, R., Cherian, J., Jauregui, J., Pierce, T., Beaver, W., and Mont, M. (2015) Genetically modified chondrocytes expressing TGF-β1: a revolutionary treatment for articular cartilage damage? Expert Opin. Biol. Ther., 15, 455-464, doi: 10.1517/14712598.2015.1009886.
Finnson, K., Chi, Y., Bou-Gharios, G., Leask, A., and Philip, A. (2012) TGF-beta signaling in cartilage homeostasis and osteoarthritis, Front. Biosci. (Schol. Ed.), 1, 251-268, doi: 10.2741/s266.
Jeuken, R., Roth, A., Peters, R., Van Donkelaar, C., Thies, J., Van Rhijn, L., and Emans, P. J. (2016) Polymers in cartilage defect repair of the knee: current status and future prospects, Polymers, 4, 8, doi: 10.3390/polym8060219.
Plánka, L., Starý, D., Srnec, R., Necas, A., and Gál, P. (2008) New options for management of posttraumatic articular cartilage defects, Rozhledy v Chirurgii : Měsíčník Československé Chirurgické Společnosti, 87, 1, 42-45.
Diederichs, S., Gabler, J., Autenrieth, J., Kynast, K., Merle, C., Walles, H., Utikal, J., and Richter, W. (2016) Differential regulation of SOX9 protein during chondrogenesis of induced pluripotent stem cells versus mesenchymal stromal cells: a shortcoming for cartilage formation, Stem Cells Dev., 25, 598-609, doi: 10.1089/scd.2015.0312.
Skuse, G., and Lamkin-Kennard, K. (2013) Reverse engineering life: physical and chemical mimetics for controlled stem cell differentiation into cardiomyocytes, Methods Mol. Biol., 1001, 99-114, doi: 10.1007/978-1-62703-363-3_9.
Tokuda, S., and Yu, A. (2019) Regulation of epithelial cell functions by the osmolality and hydrostatic pressure gradients: a possible role of the tight junction as a sensor, Intern. J. Mol. Sci., 20, doi: 10.3390/ijms20143513.
Vieira, H., Alves, P., and Vercelli, A. (2011) Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species, Prog. Neurobiol., 93, 444-455, doi: 10.1016/j.pneurobio.2011.01.007.
Khamo, J., Krishnamurthy, V., Sharum, S., Mondal, P., and Zhang, K. (2017) Applications of optobiology in intact cells and multicellular organisms, J. Mol. Biol., 429, 2999-3017, doi: 10.1016/j.jmb.2017.08.015.
Bozhokin, M. S., Bozhkova, S. A., Netyl’ko, G. I., Nakonechnyy, D. G., Blinova, M. I., and Nashchekina, Yu. A. (2018) Results of replacing superficial defect in rat hyaline cartilage by experimental cell engineering construct [in Russian], Trudy Karel’skogo NTs RAN, 4, 13-22, doi: 10.17076/them815.
Kuroda, Y., Kawai, T., Goto, K., and Matsuda, S. (2019) Clinical application of injectable growth factor for bone regeneration: a systematic review, Inflamm. Regen., 39, 1-10, doi: 10.1186/s41232-019-0109-x.
Hamann, A., Nguyen, A., and Pannier, A. (2019) Nucleic acid delivery to mesenchymal stem cells: a review of nonviral methods and applications, J. Biol. Engin., 13, 7, doi: 10.1186/s13036-019-0140-0.
Oggu, G., Sasikumar, S., Reddy, N., Ella, K., Rao, C., and Bokara, K. (2017) Gene delivery approaches for mesenchymal stem cell therapy: strategies to increase efficiency and specificity, Stem Cell Rev., 13, 725-740, doi: 10.1007/s12015-017-9760-2.
Hata, A., and Chen, Y. (2016) TGF-β signaling from receptors to Smads, Cold Spring Harb. Perspect. Biol., 8, doi: 10.1101/cshperspect.a022061.
Yang, X., Chen, L., Xu, X., Li, C., Huang, C., and Deng, C. (2001) TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage, J. Cell Biol., 153, 35-46.
Moses, H., Roberts, A., and Derynck, R. (2016) The discovery and early days of TGF-b: a historical perspective, Cold Spring Harb. Perspect. Biol., 8, doi: 10.1101/cshperspect.a021865.
Derynck, R., and Budi, E. (2019) Specificity, versatility, and control of TGF-β family signaling, Sci. Signal., 12, 570, doi: 10.1126/scisignal.aav5183.
De Larco, J., and Todaro, G. (1978) Growth factors from murine sarcoma virus-transformed cells, Proc. Natl. Acad. Sci. USA, 75, 4001-4005.
Todaro, G., De Larco, J., and Fryling, C. (1982) Sarcoma growth factor and other transforming peptides produced by human cells: interactions with membrane receptors, Fed. Proc., 41, 2996-3003.
Kastin, A. (2013) Handbook of Biologically Active Peptides, Chapter 225, doi: 10.1016/C2010-0-66490-X.
Assoian, R., Komoriya, A., Meyers, C., Miller, D., and Sporn, M. (1983) Transforming growth factor-β in human platelets. Identification of a major storage site, purification, and characterization, J. Biol. Chem., 258, 7155-7160.
Frenkel, S., Saadeh, P., Mehrara, B., Chin, G., Steinbrech, D., Brent, B., Gittes, G., and Longaker, M. (2000) Transforming growth factor beta superfamily members: role in cartilage modeling, Plast. Reconstr. Surg., 105, 980-990, doi: 10.1097/00006534-200003000-00022.
Reddi, A., and Cunningham, N. (1990) Bone induction by osteogenin and bone morphogenetic proteins, Biomaterials, 11, 33-34.
Glick, A., Weinberg, W., Wu, I., Quan, W., and Yuspa, S. (1996) Transforming growth factor beta 1 suppresses genomic instability independent of a G1 arrest, p53, and Rb, Cancer Res., 56, 3645-3650.
Massagué, J. (1999) Wounding Smad, Nat. Cell Biol., 1, 117-119, doi: 10.1038/12944.
Herpin, A., Lelong, C., and Favrel, P. (2004) Transforming growth factor-β-related proteins: an ancestral and widespread superfamily of cytokines in metazoans, Dev. Comp. Immunol., 28, 461-485, doi: 10.1016/j. dci. 2003.09.007.
Kwiatkowski, W., Gray, P., and Choe, S. (2014) Engineering TGF-β superfamily ligands for clinical applications, Trends Pharmacol. Sci., 35, 648-657, doi: 10.1016/j.tips.2014.10.006.
Lu, Y., Boer, J., Barsova, R., Favorova, O., Goel, A., Müller, M., and Feskens, E. (2012) TGFB1 genetic polymorphisms and coronary heart disease risk: a meta-analysis, BMC Med. Genet., 13, 39, doi: 10.1186/1471-2350-13-39.
Rao, K., Nagireddy, S., and Chakrabarti, S. (2011) Complex genetic mechanisms in glaucoma: an overview, Ind. J. Ophthalm., 59, 31-42, doi: 10.4103/0301-4738.73685.
Leutermann, R., Sheikhzadeh, S., Brockstädt, L., Rybczynski, M., van Rahden, V., Kutsche, K., von Kodolitsch, Y., and Rosenberger, G. (2014) A 1-bp duplication in TGFB2 in three family members with a syndromic form of thoracic aortic aneurysm, Eur. J. Hum. Genet., 22, 944-948, doi: 10.1038/ejhg.2013.252.
Occleston, N., Laverty, H., O’Kane, S., and Ferguson, M. (2008) Prevention and reduction of scarring in the skin by transforming growth factor beta 3 (TGFβ3): from laboratory discovery to clinical pharmaceutical, J. Biomater. Sci., Polymer Edition, 19, 1047-1063, doi: 10.1163/156856208784909345.
Gilbert, R., Vickaryous, M., and Viloria-Petit, A. (2016) Signalling by transforming growth factor beta isoforms in wound healing and tissue regeneration, J. Dev. Biol., 22, 4, doi: 10.3390/jdb4020021.
Furumatsu, T., Tsuda, M., Taniguchi, N., Tajima, Y., and Asahara, H. (2005) Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment, J. Biol. Chem., 280, 8343-8350, doi: 10.1074/jbc.M413913200.
Yu, J., Shao, L., Lemas, V., Yu, A., Vaughan, J., Rivier, J., and Vale, W. (1987) Importance of FSH-releasing protein and inhibin in erythrodifferentiation, Nature, 330, 765-767, doi: 10.1038/330765a0.
Bloise, E., Ciarmela, P., Cruz, C., Luisi, S., Petraglia, F., and Reis, F. (2019) Activin A in mammalian physiology, Physiol. Rev., 99, 739-780, doi: 10.1152/physrev.00002.2018.
Ling, N., Ying, S., Ueno, N., Shimasaki, S., Esch, F., Hotta, M., and Guillemin, R. (1986) Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin, Nature, 321, 779-782, doi: 10.1038/321779a0.
Namwanje, M., and Brown, C. (2016) Activins and inhibins: roles in development, physiology, and disease, Cold Spring Harb. Perspect. Biol., 8, doi: 10.1101/cshperspect.a021881.
Chin, D., Boyl, G., Parsons, P., and Coman, W. (2004) What is transforming growth factor-beta (TGF-β)? Brit. J. Plastic Surg., 57, 215-221, doi: 10.1016/j.bjps.2003.12.012.
Kushnir, V., Seifer, D., Barad, D., Sen, A., and Gleicher, N. (2017) Potential therapeutic applications of human anti-Müllerian hormone (AMH) analogues in reproductive medicine, J. Assist. Reprod. Genet., 34, 1105-1113, doi: 10.1007/s10815-017-0977-4.
Tabibzadeh, S., and Hemmati-Brivanlou, A. (2006) Lefty at the crossroads of “stemness” and differentiative events, Stem Cells, 24, 1998-2006, doi: 10.1634/stemcells.2006-0075.
Jones, C., Kuehn, M., Hogan, B., Smith, J., and Wright, C. (1995) Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation, Development, 121, 3651-3662.
Morikawa, M., Derynck, R., and Miyazono, K. (2016) TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology, Cold Spring Harb. Perspect. Biol., 2, 8, doi: 10.1101/cshperspect.a021873.
Papageorgis, P., and Stylianopoulos, T. (2015) Role of TGFβ in regulation of the tumor microenvironment and drug delivery (review), Int. J. Oncol., 46, 933-943, doi: 10.3892/ijo.2015.2816.
Gatza, C., Oh, S., and Blobe, G. (2010) Roles for the type III TGF-β receptor in human cancer, Cell. Signal., 22, 1163-1174, doi: 10.1016/j.cellsig.2010.01.016.
Lawler, S., Feng, X., Chen, R., Maruoka, E., Turck, C., Griswold-Prenner, I., and Derynck, R. (1997) The type II transforming growth factor-β receptor autophosphorylates not only on serine and threonine but also on tyrosine residues, J. Biol. Chem., 272, 14850-14859, doi: 10.1074/jbc.272.23.14850.
Heldin, C., and Moustakas, A. (2016) Signaling receptors for TGF-β family members, Cold Spring Harb. Perspect. Biol., 8, 8, doi: 10.1101/cshperspect.a022053.
Ahmadi, A., Najafi, M., Farhood, B., and Mortezaee, K. (2019) Transforming growth factor-β signaling: tumorigenesis and targeting for cancer therapy, J. Cell. Physiol., 234, 12173-12187, doi: 10.1002/jcp.27955.
Wrana, J., Attisano, L., Cárcamo, J., Zentella, A., Doody, J., Laiho, M., Wang, X., and Massagué, J. (1992) TGFβ signals through a heteromeric protein kinase receptor complex, Cell, 71, 1003-1014, doi: 10.1016/0092-8674(92)90395-S.
Yamashita, H., ten Dijke, P., Franzén, P., Miyazono, K., and Heldin, C. (1994) Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β, J. Biol. Chem., 269, 20172-20178.
Massagué, J. (1998) TGF-beta signal transduction, Annu. Rev. Biochem., 67, 753-791, doi: 10.1146/annurev.biochem.67.1.753.
Massagué, J., and Chen, Y. (2000) Controlling TGF-beta signaling, Genes Dev., 14, 627-644.
Feng, X., and Derynck, R. (2005) Specificity and versatility in TGF-beta signaling through Smads, Annu. Rev. Cell Dev. Biol., 21, 659-693, doi: 10.1146/annurev.cellbio.21.022404.142018.
Huang, T., David, L., Mendoza, V., Yang, Y., Villarreal, M., De, K., Sun, L., Fang, X., López-Casillas, F., Wrana, J., and Hinck, A. (2011) TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs, EMBO J., 30, 1263-1276, doi: 10.1038/emboj.2011.54.
Atfi, A., Dumont, E., Colland, F., Bonnier, D., L’helgoualc’h, A., Prunier, C., Ferrand, N., Clément, B., Wewer, U., and Théret, N. (2007) The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor, J. Cell Biol., 16, 201-208, doi: 10.1083/jcb.200612046.
Kang, J., Liu, C., and Derynck, R. (2009) New regulatory mechanisms of TGF-β receptor function, Trends Cell Biol., 19, 385-394, doi: 10.1016/j.tcb.2009.05.008.
Imamura, T., Oshima, Y., and Hikita, A. (2013) Regulation of TGF-β family signalling by ubiquitination and deubiquitination, J. Biochem., 154, 481-489, doi: 10.1093/jb/mvt097.
Zuo, W., Huang, F., Chiang, Y., Li, M., Du, J., Ding, Y., Zhang, T., Lee, H., Jeong, L., Chen, Y., Deng, H., Feng, X., Luo, S., Gao, C., and Chen, Y. (2013) c-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-β type II receptor, Mol. Cell, 49, 499-510, doi: 10.1016/j.molcel.2012.12.002.
Zhang, L., Zhou, F., Drabsch, Y., Gao, R., Snaar-Jagalska, B., Mickanin, C., Huang, H., Sheppard, K., Porter, J., Lu, C., and Dijke, P. (2012) USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor, Nat. Cell Biol., 14, 717-726, doi: 10.1038/ncb2522.
Flotho, A., and Melchior, F. (2013) Sumoylation: a regulatory protein modification in health and disease, Annu. Rev. Biochem., 82, 357-385, doi: 10.1146/annurev-biochem-061909-093311.
Mu, Y., Sundar, R., Thakur, N., Ekman, M., Gudey, S., Yakymovych, M., Hermansson, A., Dimitriou, H., Bengoechea-Alonso, M., Ericsson, J., Heldin, C., and Landström, M. (2011) TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer, Nat. Commun., 2, 330, doi: 10.1038/ncomms1332.
Li, S. B., and Wu, J. F. (2020) TGF-β/SMAD Signaling regulation of mesenchymal stem cells in adipocyte commitment, Rev. Stem Cell Res. Therl., 11, 41, doi: 10.1186/s13287-020-1552-y.
Nishita, M., Ueno, N., and Shibuya, H. (1999) Smad8B, a Smad8 splice variant lacking the SSXS site that inhibits Smad8-mediated signaling, Genes Cells, 4, 583-591, doi: 10.1046/j.1365-2443.1999.00285.x.
Hill, C. (2009) Nucleocytoplasmic shuttling of Smad proteins, Cell Res., 19, 36-46, doi: 10.1038/cr.2008.325.
Wu, M., Chen, G., and Li, Y. (2016) TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res., 4, 16009, doi: 10.1038/boneres.2016.9.
Kang, J., Alliston, T., Delston, R., and Derynck, R. (2014) Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3, EMBO J., 24, 2543-2555.
Massagué, J. (2014) TGFbeta signalling in context, Nat. Rev. Mol. Cell Biol., 13, 616-630, doi: 10.1038/nrm3434.
Zhang, S., Fei, T., Zhang, L., Zhang, R., Chen, F., Ning, Y., Han, Y., Feng, X., Meng, A., and Chen, Y. (2007) Smad7 antagonizes transforming growth factor signaling in the nucleus by interfering with functional Smad–DNA complex formation, Mol. Cell. Biol., 27, 4488-4499, doi: 10.1128/mcb.01636-06.
Gu, W., Monteiro, R., Zuo, J., Simões, F., Martella, A., Andrieu-Soler, C., Grosveld, F., Sauka-Spengler, T., and Patient, R. (2015) A novel TGFβ modulator that uncouples R-Smad/I-Smad-mediated negative feedback from R-Smad/ligand-driven positive feedback, PLoS Biol., 13, doi: 10.1371/journal.pbio.1002051.
Thielen, N., van der Kraan, P., and van Caam, A. (2019) TGFβ/BMP signaling pathway in cartilage homeostasis, Cells, 8, 9, doi: 10.3390/cells8090969.
Li, Y., Luo, W., and Yang, W. (2018) Nuclear transport and accumulation of Smad proteins studied by single-molecule microscopy, Biophys. J., 114, 2243-2251, doi: 10.1016/j.bpj.2018.03.018.
Jin, Q., Gao, G., and Mulder, K. (2009) Requirement of a dynein light chain in TGFβ/Smad3 signaling, J. Cell. Physiol., 221, 707-715, doi: 10.1002/jcp.21910.
Batut, J., Howell, M., and Hill, C. (2007) Kinesin-mediated transport of Smad2 is required for signaling in response to TGF-β ligands, Dev. Cell., 12, 261-274, doi: 10.1016/j.devcel.2007.01.010.
Massagué, J., Seoane, J., and Wotton, D. (2005) Smad transcription factors, Genes Dev., 19, 2783-2810, doi: 10.1101/gad.1350705.
Hill, C. (2016) Transcriptional control by the SMADs, Cold Spring Harb. Perspect. Biol., 8, doi: 10.1101/cshperspect.a022079.
Qiao, B., Padilla, S., and Benya, P. (2005) Transforming growth factor (TGF)-β-activated kinase 1 mimics and mediates TGF-β-induced stimulation of type II collagen synthesis in chondrocytes independent of Col2a1 transcription and Smad3 signaling, J. Biol. Chem., 280, 17562-17571, doi: 10.1074/jbc.M500646200.
Bhogal, R., Stoica, C., McGaha, T., and Bona, C. (2005) Molecular aspects of regulation of collagen gene expression in fibrosis, J. Clin. Immun., 25, 592-603, doi: 10.1007/s10875-005-7827-3.
Bell, D., Leung, K., Wheatley, S., Ng, L., Zhou, S., Ling, K., Sham, M., Koopman, P., Tam, P., and Cheah, K. (1997) SOX9 directly regulates the type-II collagen gene, Nat. Genet., 16, 174-178, doi: 10.1038/ng0697-174.
Sen, R., Pezoa, S., Carpio, Shull, L., Hernandez-Lagunas, L., Niswander, L., and Artinger, K. (2018) Kat2a and Kat2b acetyltransferase activity regulates craniofacial cartilage and bone differentiation in Zebrafish and mice, J. Dev. Biol., 12, doi: 10.3390/jdb6040027.
Chen, X., Huang, H., Wang, H., Guo, F., Du, X., Ma, L., Zhao, L., Pan, Z., Gui, H., Yuan, T., Liu, X., Song, L., Wang, Y., He, J., Lei, H., and Gao, R. (2014) Characterization of zebrafish pax1b and pax9 in fin bud development, Biomed. Res. Int., 2014, 309385, doi: 10.1155/2014/309385.
Seoane, J., Le, H., Shen, L., Anderson, S., and Massagué, J. (2004) Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation, Cell, 117, 211-223, doi: 10.1016/S0092-8674(04)00298-3.
Naka, K., Hoshii, T., Muraguchi, T., Tadokoro, Y., Ooshio, T., Kondo, Y., Nakao, S., Motoyama, N., and Hirao, A. (2010) TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia, Nature, 463, 676-680, doi: 10.1038/nature08734.
Kang, Y., Chen, C., and Massagué, J. (2003) A self-enabling TGFβ response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells, Mol. Cell, 11, 915-926, doi: 10.1016/S1097-2765(03)00109-6.
Vincent, T., Neve, E., Johnson, J., Kukalev, A., Rojo, F., Albanell, J., Pietras, K., Virtanen, I., Philipson, L., Leopold, P., Crystal, R., de Herreros, A., Moustakas, A., Pettersson, R., and Fuxe, J. (2009) A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial-mesenchymal transition, Nat. Cell Biol., 11, 943-950, doi: 10.1038/ncb1905.
Pardali, K., Kurisaki, A., Morén, A., ten Dijke, P., Kardassis, D., and Moustakas, A. (2000) Role of Smad proteins and transcription factor Sp1 in p21Waf1/Cip1 regulation by transforming growth factor-β, J. Biol. Chem., 275, 29244-29256, doi: 10.1074/jbc.M909467199.
Baugé, C., Cauvard, O., Leclercq, S., Galéra, P., and Boumédiene, K. (2011) Modulation of transforming growth factor beta signalling pathway genes by transforming growth factor beta in human osteoarthritic chondrocytes: Involvement of Sp1 in both early and late response cells to transforming growth factor beta, Arthritis Res. Ther., 13, 23, doi: 10.1186/ar3247.
Blaney Davidson, E., Remst, D. F., Vitters, E., van Beuningen, H., Blom, A., Goumans, M., van den Berg, W., and van der Kraan, P. (2009) Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice, J. Immunol., 182, 7937-7945, doi: 10.4049/jimmunol.0803991.
Siomi, H., and Siomi, M. (2010) Posttranscriptional regulation of microRNA biogenesis in animals, Mol. Cell, 38, 323-332, doi: 10.1016/j.molcel.2010.03.013.
Ha, M., and Kim, V. (2014) Regulation of microRNA biogenesis, Nat. Rev. Mol. Cell Biol., 15, 509-524, doi: 10.1038/nrm3838.
Blahna, M., and Hata, A. (2012) Smad-mediated regulation of microRNA biosynthesis, FEBS Lett., 586, 1906-1912, doi: 10.1016/j.febslet.2012.01.041.
Zhang, Y., Huang, X., and Yuan, Y. (2017) MicroRNA-410 promotes chondrogenic differentiation of human bone marrow mesenchymal stem cells through down-regulating Wnt3a, Am. J. Transl. Res., 9, 136-145.
Lee, S., Yoon, D., Paik, S., Lee, K., Jang, Y., and Lee, J. (2014) MicroRNA-495 Inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9, Stem Cells Dev., 23, 1798-1808, doi: 10.1089/scd.2013.0609.
Crecente-Campo, J., Borrajo, E., Vidal, A., and Garcia-Fuentes, M. (2017) New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation, Eur. J. Pharm. Biopharm., 114, 69-78, doi: 10.1016/j.ejpb.2016.12.021.
Wang, J., Sun, B., Tian, L., He, X., Gao, Q., Wu, T., Ramakrishna, S., Zheng, J., and Mo, X. (2017) Evaluation of the potential of rhTGF-β3 encapsulated P(LLA-CL)/collagen nanofibers for tracheal cartilage regeneration using mesenchymal stems cells derived from Wharton’s jelly of human umbilical cord, Mater. Sci. Eng., 70, 637-645, doi: 10.1016/j.msec.2016.09.044.
Yanagawa, Y., Hiraide, S., and Iizuka, K. (2016) Isoform-specific regulation of transforming growth factor-β mRNA expression in macrophages in response to adrenoceptor stimulation, Microbiol. Immunol., 60, 56-63, doi: 10.1111/1348-0421.12344.
Frangogiannis, N. G. (2017) The role of transforming growth factor (TGF)-β in the infarcted myocardium, J. Thor. Dis., 9, 52-63, doi: 10.21037/jtd.2016.11.19.
Luo, Z., Jiang, L., Xu, Y., Li, H., Xu, W., Wu, S., Wang, Y., Tang, Z., Lv, Y., and Yang, L. (2015) Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model, Biomaterials, 52, 463-475, doi: 10.1016/j.biomaterials.2015.01.001.
Yang, S. S., Jin, L. H., Park, S. H., Kim, M. S., Kim, Y. J., Choi, B. H., Lee, C. T., Park, S. R., and Min, B. H. (2016) Extracellular matrix (ECM) multilayer membrane as a sustained releasing growth factor delivery system for rhTGF-β3 in articular cartilage repair, PLoS One, 11, e0156292, doi: 10.1371/journal.pone.0156292.
Yang, Q., Teng, B. H., Wang, L. N., Li, K., Xu, C., Ma, X. L., Zhang, Y., Kong, D. L., Wang, L. Y., and Zhao, Y. H. (2017) Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells, Int. J. Nanomed., 12, 6721-6733, doi: 10.2147/IJN.S141888.
Wang, X., Li, Y., Han, R., He, C., Wang, G., Wang, J., Zheng, J., Pei, M., and Wei, L. (2014) Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-β3 gene promoted pig cartilage defect repair, PLoS One, 9, e116061, doi: 10.1371/journal.pone.0116061.
Sun, Q., Zhang, L., Xu, T., Ying, J., Xia, B., Jing, H., and Tong, P. (2018) Combined use of adipose derived stem cells and TGF-β3 microspheres promotes articular cartilage regeneration in vivo, Biotech. Histochem., 93, 168-176, doi: 10.1080/10520295.2017.1401663.
Zhou, M., Lozano, N., Wychowaniec, J. K., Hodgkinson, T., Richardson, S. M., Kostarelos, K., and Hoyland, J. A. (2019) Graphene oxide: a growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels, Acta Biomater., 96, 271-280, doi: 10.1016/j.actbio.2019.07.027.
Rothrauff, B., Sasaki, H., Kihara, S., Overholt, K., Gottardi, R., Lin, H., Fu, F., Tuan, R., and Alexander, P. (2019) Point-of-care procedure for enhancement of meniscal healing in a goat model utilizing infrapatellar fat pad-derived stromal vascular fraction cells seeded in photocrosslinkable hydrogel, Am. J. Sports Med., 47, 3396-3405, doi: 10.1177/0363546519880468.
Acknowledgements
We thank the Chromas Shared Use Center, Biobank, Center for Molecular and Cell Technologies, and Research Park of Saint Petersburg State University for collaboration.
Funding
This study was performed within the framework of the Russian Academic State Task for the Institute of Cytology with financial support from the Ministry of Education and Science of the Russian Federation and the Saint-Petersburg State University (grant ID 51140332).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies involving animals or human participants performed by any of the authors. The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bozhokin, M., Sopova, Y., Kachkin, D. et al. Mechanisms of TGFβ3 Action as a Therapeutic Agent for Promoting the Synthesis of Extracellular Matrix Proteins in Hyaline Cartilage. Biochemistry Moscow 85, 436–447 (2020). https://doi.org/10.1134/S0006297920040045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920040045