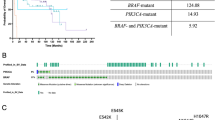
Abstract
The MAPK (RAS/BRAF/MEK/ERK) signaling pathway is a kinase cascade involved in the regulation of cell proliferation, differentiation, and survival in response to external stimuli. The V600E mutation in the BRAF gene has been detected in various tumors, resulting in a 500-fold increase in BRAF kinase activity. However, monotherapy with selective BRAF V600E inhibitors often leads to reactivation of MAPK signaling cascade and emergence of drug resistance. Therefore, new targets are being developed for the inhibition of components of the aberrantly activated cascade. It was recently discovered that resistance to BRAF V600E inhibitors may be associated with the activity of the tyrosine phosphatase SHP-2 encoded by the PTPN11 gene. In this paper, we analyzed transcriptional effects of PTPN11 gene knockdown and selective suppression of BRAF V600E in a model of thyroid follicular epithelium. We found that the siRNA-mediated knockdown of PTPN11 after vemurafenib treatment prevented an increase in the expression CCNA1 and NOTCH4 genes involved in the formation of drug resistance of tumors. On the other hand, downregulation of PTPN11 expression blocked the transcriptional activation of genes (p21, pl5, pl6, RBI, and IGFBP7) involved in cell cycle regulation and oncogene-induced senescence in response to BRAF V600E expression. Therefore, it can be assumed that SHP-2 participates not only in emergence of drug resistance in cancer cells, but also in oncogene-induced cell senescence.
Similar content being viewed by others
Abbreviations
- EGFR:
-
epidermal growth factor receptor
- OIS:
-
oncogene-induced senescence
- RT-qPCR:
-
reverse transcription/quantitative PCR.
References
Burotto, M., Chiou, V. L., Lee, J. M., and Kohn, E. C. (2014) The MAPK pathway across different malignancies: a new perspective, Cancer, 120, 3446–3456, doi: https://doi.org/10.1002/cncr.28864.
Davies, H., Bignell, G. R., Cox, C., Stephens, P., Edkins, S., et al. (2002) Mutations of the BRAF gene in human cancer, Nature, 417, 949–954, doi: https://doi.org/10.1038/nature00766.
Mesa, C., Jr., Mirza, M., Mitsutake, N., Sartor, M., Medvedovic, M., Tomlinson, C., Knauf, J. A., Weber, G. E., and Fagin, J. A. (2006) Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling, Cancer Res., 66, 6521–6529, doi: https://doi.org/10.1158/0008-5472.CAN-06-0739.
Long, G. V., Menzies, A. M., Nagrial, A. M., Haydu, L. E., Hamilton, A. L., Mann, G. J., Hughes, T. M., Thompson, J. F., Scolyer, R. A., and Kefford, R. E (2011) Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma, J. Clin. Oncol, 29, 1239–1246, doi: https://doi.org/10.1200/JCO.2010.32.4327.
Loh, M. L., Vattikuti, S., Schubbert, S., Reynolds, M. G., Carlson, E., Lieuw, K. H., Cheng, J. W., Lee, C. M., Stokoe, D., Bonifas, J. M., Curtiss, N. P., Gotlib, J., Meshinchi, S., Le Beau, M. M., Emanuel, P. D., and Shannon, K. M. (2004) Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis, Blood, 103, 2325–2331, doi: https://doi.org/10.1182/blood-2003-09-3287.
Mohi, M. G., Williams, I. R., Dearolf, C. R., Chan, G., Kutok, J. L., Cohen, S., Morgan, K., Boulton, C., Shigematsu, H., Keilhack, H., Akashi, K., Gilliland, D. G., and Neel, B. G. (2005) Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations, Cancer Cell, 7, 179–191, doi: https://doi.org/10.1016/j.ccr.2005.01.010.
Tartaglia, M., Mehler, E. L., Goldberg, R., Zampino, G., Brunner, H. G., Kremer, H., van der Burgt, I., Crosby, A. H., Ion, A., Jeffery S., Kalidas, K., Patton, M. A., Kucherlapati, R. S., and Gelb, B. D. (2001) Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome, Nat. Genet., 29, 465–468, doi: https://doi.org/10.1038/ng772.
Chan, G., Kalaitzidis, D., and Neel, B. G. (2008) The tyrosine phosphatase Shp2 (PTPN11) in cancer, Cancer Metastasis Rev., 27, 179–192, doi: https://doi.org/10.1007/sl0555-008-9126-y
Chan, R. J., and Feng, G. S. (2007) PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase, Blood, 109, 862–867, doi: https://doi.org/10.1182/blood-2006-07-028829.
Li, S. M. (2016) The biological function of SHP2 in human disease, Mol. Biol. (Moscow), 50, 27–33, doi: https://doi.org/10.7868/S0026898416010110.
Matozaki, T., Murata, Y., Saito, Y., Okazawa, H., and Ohnishi, H. (2009) Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation, Cancer Sci., 100, 1786–1793, doi: https://doi.org/10.1111/j.1349-7006.2009.01257.x.
Zhang, S. Q., Tsiaras, W.G., Araki, T., Wen, G., Minichiello, L., Klein, R., and Neel, B. G. (2002) Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2, Mol. Cell. Biol., 11, 4062–4072.
Saxton, T. M., Henkemeyer, M., Gasca, S., Shen, R., Rossi, D. J., Shalaby F., Feng, G. S., and Pawson, T. (1997) Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2, EMBO J., 16, 2352–2364, doi: https://doi.org/10.1093/emboj/16.9.2352.
Tajan, M., Batut, A., Cadoudal, T., Deleruyelle, S., Le Gonidec, S., Saint Laurent, C., Vomscheid, M., Wanecq, E., Treguer, K., De Rocca Serra-Nedelec, A, Vinel, C., Marques, M. A., Pozzo, J., Kunduzova, O., Salles, J. P., Tauber, M., Raynal, P., Cave, H., Edouard, T, Valet, P., and Yart, A. (2014) LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity, Proc. Natl. Acad. Sci. USA, 111, E4494–E4503, doi: https://doi.org/10.1073/pnas.1406107111.
Chen, L., Chen, W, Mysliwski, M., Serio, J., Ropa, J., Abulwerdi, F A., Chan, R. J., Patel, J. P., Tallman, M. S., Paietta, E., Melnick, A., Levine, R. L., Abdel-Wahab, O., Nikolovska-Coleska, Z., and Muntean, A. G. (2015) Mutated Ptpnll alters leukemic stem cell frequency and reduces the sensitivity of acute myeloid leukemia cells to Mcll inhibition, Leukemia, 29, 1290–1300, doi: https://doi.org/10.1038/leu.2015.18.
Qu, C. K., Shi, Z. Q., Shen, R., Tsai, F Y, Orkin, S. H., and Feng, G. S. (1997) A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development, Mol. Cell. Biol., 17, 5499–5507.
Bard-Chapeau, E. A., Li, S., Ding, J., Zhang, S. S., Zhu, H. H., Princen, F., Fang, D. D., Han, T., Bailly-Maitre, B., Poli, V, Varki, N. M., Wang, H., and Feng, G. S. (2011) Ptpnll/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis, Cancer Cell, 19, 629–639, doi: https://doi.org/10.1016/j.ccr.2011.03.023.
Yang, W., Wang, J., Moore, D. C., Liang, H., Dooner, M., Wu, Q., Terek, R., Chen, Q., Ehrlich, M. G., Quesenberry P. J., and Neel, B. G. (2013) Ptpnll deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling, Nature, 499, 491–495, doi: https://doi.org/10.1038/naturel2396.
Hill, K. S., Roberts, E. R., Wang, X., Marin, E., Park, T. D., Son, S., Ren, Y., Fang, B., Yoder, S., Kim, S., Wan, L., Sarnaik, A. A, Koomen, J. M., Messina, J. L., Teer, J. K., Kim, Y, Wu, J., Chalfant, C. E., and Kim, M. (2019) PTPN11 plays oncogenic roles and is a therapeutic target for BRAF wild-type melanomas, Mol. Cancer Res., 17, 583–593, doi: https://doi.org/10.1158/1541-7786.MCR-18-0777.
Zhan, Y., Counelis, G. J., and O’Rourke, D. M. (2009) The protein tyrosine phosphatase SHP-2 is required for EGFRvIII oncogenic transformation in human glioblastoma cells, Exp. Cell Res., 315, 2343–2357, doi: https://doi.org/10.1016/j.yexcr.2009.05.001.
Hu, Z. Q., Ma, R., Zhang, C. M., Li, J., Li, L., Hu, Z. T., Gao, Q. I., and Li, W. M. (2015) Expression and clinical significance of tyrosine phosphatase SHP2 in thyroid carcinoma, Oncol. Lett., 10, 1507–1512, doi: https://doi.org/10.3892/ol.2015.3479.
Prahallad, A., Heynen, G. J., Germano, G., Willems, S. M., Evers, B., Vecchione, L., Gambino, V, Lieftink, C, Beijersbergen, R. L., Di Nicolantonio, E, Bardelli, A., and Bernards, R. (2015) PTPN11 is a central node in intrinsic and acquired resistance to targeted cancer drugs, Cell Rep., 12, 1978–1985, doi: https://doi.org/10.1016/j.celrep.2015.08.037.
Schneeberger, V. E., Ren, Y., Luetteke, N., Huang, Q., Chen, L., Lawrence, H. R., Lawrence, N. J., Haura, E. B., Koomen, J. M., Coppola, D., and Wu, J. (2015) Inhibition of Shp2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma, Oncotarget, 6, 6191–6202, doi: https://doi.org/10.18632/oncotarget.3356.
Chen, Y. N., LaMarche, M. J., Chan, H. M., Fekkes, P., Garcia-Fortanet, J., et al. (2016) Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases, Nature, 535, 148–152, doi: https://doi.org/10.1038/nature18621.
Chapman, P. B., Hauschild, A., Robert, C., Haanen, J. B., Ascierto, P., et al. (2011) Improved survival with vemu-rafenib in melanoma with BRAF V600E mutation, N. Engl. J. Med., 364, 2507–2516, doi: https://doi.org/10.1056/NEJMoall03782.
Montero-Conde, C., Ruiz-Llorente, S., Dominguez, J. M., Knauf, J. A., Viale, A., Sherman, E. J., Ryder, M., Ghossein, R. A., Rosen, N., and Fagin, J. A. (2013) Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas, Cancer Discov., 3, 520–533, doi: https://doi.org/10.1158/2159-8290.CD-12-0531.
Nazarian, R., Shi, H., Wang, Q., Kong, X., Koya, R. C., Lee, H., Chen, Z., Lee, M. K., Attar, N., Sazegar, H., Chodon, T., Nelson, S. F, McArthur, G., Sosman, J. A, Ribas, A., and Lo, R. S. (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation, Nature, 468, 973–977, doi: https://doi.org/10.1038/nature09626.
Boehm, J. S., Zhao, J. J., Yao, J., Kim, S. Y., Firestein, R., Dunn, I. F., Sjostrom, S. K., Garraway L. A., Weremowicz, S., Richardson, A. L., Greulich, H., Stewart, C. J., Mulvey L. A., Shen, R. R., Ambrogio, L., Hirozane-Kishikawa, T, Hill, D. E., Vidal, M., Meyerson, M., Grenier, J. K., Hinkle, G., Root, D. E., Roberts, T. M., Lander, E. S., Polyak, K., and Hahn, W. C. (2007) Integrative genomic approaches identify IKBKE as a breast cancer oncogene, Cell, 129, 1065–1079, doi: https://doi.org/10.1016/j.cell.2007.03.052.
Prokofjeva, M. M., Proshkina, G. M., Lebedev, T. D., Shulgin, A. A., Spirin, P. V., Prassolov, V. S., and Deyev, S. M. (2017) Lentiviral gene delivery to plasmolipin-express-ing cells using Mus caroli endogenous retrovirus envelope protein, Biochimie, 142, 226–233, doi: https://doi.org/10.1016/j.biochi.2017.09.004.
Liu, Z., Zhao, Y., Fang, J., Cui, R., Xiao, Y., and Xu, Q. (2017) SHP2 negatively regulates HLA-ABC and PD-L1 expression via STAT1 phosphorylation in prostate cancer cells, Oncotarget, 8, 53518–53530, doi: https://doi.org/10.18632/oncotarget.18591.
Schwartz, A. M., Putlyaeva, L. V., Covich, M., Klepikova, A. V., Akulich, K. A., Vorontsov, I. E., Korneev, K. V, Dmitriev, S. E., Polanovsky O. L., Sidorenko, S. P., Kulakovskiy I. V, and Kuprash, D. V. (2016) Early B-cell factor 1 (EBF1) is critical for transcriptional control of SLAMF1 gene in human B cells, Biochim. Biophys. Acta, 1859, 1259–1268, doi: https://doi.org/10.1016/j.bbagrm.2016.07.004.
Afanasyeva, M. A., Britanova, L. V., Korneev, K. V., Mitkin, N. A., Kuchmiy A. A., and Kuprash, D. V. (2014) Clusterin is a potential lymphotoxin beta receptor target that is upregulated and accumulates in germinal centers of mouse spleen during immune response, PLoS One, 9, e98349, doi: https://doi.org/10.1371/journal.pone.0098349.
Bolger, A. M., Lohse, M., and Usadel, B. (2014) Trimmomatic: a flexible trimmer for Illumina sequence data, Bioinformatics, 30, 2114–2120, doi: https://doi.org/10.1093/bioinformatics/btul70.
Dobin, A., Davis, C. A., Schlesinger, E., Drenkow, J., Zaleski, C., Jha, S., Batut, P., Chaisson, M., and Gingeras, T R. (2013) STAR: ultrafast universal RNA-seq aligner, Bioinformatics, 29, 15–21, doi: https://doi.org/10.1093/bioinformatics/bts635.
Liao, Y., Smyth, G. K., and Shi, W. (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features, Bioinformatics, 30, 923–930, doi: https://doi.org/10.1093/bioinformatics/btt656.
Love, M. I., Huber, W., and Anders, S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2, Genome Biol., 15, 550, doi: https://doi.org/10.1186/s13059-014-0550-8.
Zhou, Y., Zhou, B., Pache, L., Chang, M., Khodabakhshi, A. H., Tanaseichuk, O., Benner, C, and Chanda, S. K. (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets, Nat. Commun., 10, 1523, doi: https://doi.org/10.1038/s41467-019-09234-6.
Castellone, M. D., De Falco, V., Rao, D. M., Bellelli, R., Muthu, M., Basolo, F., Fusco, A., Gutkind, J. S., and Santoro, M. (2009) The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation, Cancer Res., 69, 1867–1876, doi: https://doi.org/10.1158/0008-5472.CAN-08-1982.
Giordano, T. J., Kuick, R., Thomas, D. G., Misek, D. E., Vinco, M., Sanders, D., Zhu, Z., Ciampi, R., Roh, M., Shedden, K., Gauger, P., Doherty G., Thompson, N. W., Hanash, S., Koenig, R. J., and Nikiforov, Y E. (2005) Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis, Oncogene, 24, 6646–6656, doi: https://doi.org/10.1038/sj.onc.1208822.
Nucera, C., Porrello, A., Antonello, Z. A., Mekel, M., Nehs, M. A., Giordano, T. J., Gerald, D., Benjamin, L. E., Priolo, C, Puxeddu, E., Finn, S., Jarzab, B., Hodin, R. A., Pontecorvi, A., Nose, V, Lawler, J., and Parangi, S. (2010) B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression, Proc. Natl. Acad. Sci. USA, 107, 10649–10654, doi: https://doi.org/10.1073/pnas.l004934107.
Roskoski, R., Jr. (2012) ERK1/2 MAP kinases: structure, function, and regulation, Pharmacol. Res., 66, 105–143, doi: https://doi.org/10.1016/j.phrs.2012.04.005.
Kim, B. A., Jee, H. G., Yi, J. W., Kim, S. J., Chai, Y. J., Choi, J. Y., and Lee, K. E. (2017) Expression profiling of a human thyroid cell line stably expressing the BRAF V600E mutation, Cancer Genom. Proteomics, 14, 53–67, doi: https://doi.org/10.21873/cgp.20018.
Coppe, J. P., Desprez, P. Y., Krtolica, A., and Campisi, J. (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression, Annu. Rev. Pathol, 5, 99–118, doi: https://doi.org/10.1146/annurev-pathol-121808-102144.
Hardy, K. M., Kirschmann, D. A., Seftor, E. A., Margaryan, N. V, Postovit, L. M., Strizzi, L., and Hendrix, M. J. (2010) Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype, Cancer Res., 70, 10340–10350, doi: https://doi.org/10.1158/0008-5472.CAN-10-0705.
Kim, Y. H., Choi, Y. W., Han, J. H., Lee, J., Soh, E. Y., Park, S. H., Kim, J. H., and Park, T. J. (2014) TSH signaling overcomes B-RafV600E-induced senescence in papillary thyroid carcinogenesis through regulation of DUSP6, Neoplasia, 16, 1107–1120, doi: https://doi.org/10.1016/j.neo.2014.10.005.
Moulana, F. I., Priyani, A. A. H., de Silva, M. V. C., and Dassanayake, R. S. (2018) BRAF-oncogene-induced senescence and the role of thyroid-stimulating hormone signaling in the progression of papillary thyroid carcinoma, Horm. Cancer, 9, 1–11, doi: https://doi.org/10.1007/sl2672-017-0315-4.
Simoes, B. M., O’Brien, C. S., Eyre, R., Silva, A., Yu, L., Sarmiento-Castro, A., Alferez, D. G., Spence, K., Santiago-Gomez A., Chemi, F, Acar, A., Gandhi, A, Howell, A., Brennan, K., Ryden, L., Catalano, S., Ando, S., Gee, J., Ucar, A., Sims, A. H., Marangoni, E., Farnie, G., Landberg, G., Howell, S. J., and Clarke, R. B. (2015) Anti-estrogen resistance in human breast tumors is driven by JAGl-NOTCH4-dependent cancer stem cell activity, Cell Rep., 12, 1968–1977, doi: https://doi.org/10.1016/j.celrep.2015.08.050.
Huang, K. C., Yang, J., Ng, M. C., Ng, S. K., Welch, W. R., Muto, M. G., Berkowitz, R. S., and Ng, S. W. (2016) Cyclin Al expression and paclitaxel resistance in human ovarian cancer cells, Eur. J. Cancer, 67, 152–163, doi: https://doi.org/10.1016/j.ejca.2016.08.007.
Liao, C., Wang, X. Y., Wei, H. Q., Li, S. Q., Merghoub, T., Pandolfi, P. P., and Wolgemuth, D. J. (2001) Altered myelopoiesis and the development of acute myeloid leukemia in transgenic mice overexpressing cyclin Al, Proc. Natl. Acad. Sci. USA, 98, 6853–6858, doi: https://doi.org/10.1073/pnas.121540098.
Valladares, A., Hernandez, N. G., Gomez, F S., Curiel-Quezada, E., Madrigal-Bujaidar, E., Vergara, M. D., Martinez, M. S., and Arenas Aranda, D. J. (2006) Genetic expression profiles and chromosomal alterations in sporadic breast cancer in Mexican women, Cancer Genet. Cytogenet., 170, 147–151, doi: https://doi.org/10.1016/j.cancergencyto.2006.06.002.
Takashima, S., Saito, H., Takahashi, N., Imai, K., Kudo, S., Atari, M., Saito, Y., Motoyama, S., and Minamiya, Y (2014) Strong expression of cyclin B2 mRNA correlates with a poor prognosis in patients with non-small cell lung cancer, Tumour Biol., 35, 4257–4265, doi: https://doi.org/10.1007/sl3277-013-1556-7.
Ahmed, T. A., Adamopoulos, C., Karoulia, Z., Wu, X., Sachidanandam, R., Aaronson, S. A., and Poulikakos, P. I. (2019) SHP2 drives adaptive resistance to ERK signaling inhibition in molecularly defined subsets of ERK-depend-ent tumors, Cell Rep., 26, 65–78 e65, doi: https://doi.org/10.1016/j.celrep.2018.12.013.
Corcoran, R. B., Ebi, H., Turke, A. B., Coffee, E. M., Nishino, M., Cogdill, A. P., Brown, R. D., Delia Pelle, P., Dias-Santagata, D., Hung, K. E., Flaherty, K. T, Piris, A., Wargo, J. A., Settleman, J., Mino-Kenudson, M., and Engelman, J. A. (2012) EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemu-rafenib, Cancer Discov., 2, 227–235, doi: https://doi.org/10.1158/2159-8290.CD-11-0341.
Duncan, J. S., Whittle, M. C., Nakamura, K., Abell, A. N., Midland, A. A., Zawistowski, J. S., Johnson, N. L., Granger, D. A, Jordan, N. V, Darr, D. B., Usary J., Kuan, P. F, Smalley D. M., Major, B., He, X., Hoadley K. A., Zhou, B., Sharpless, N. E., Perou, C. M., Kim, W Y, Gomez, S. M., Chen, X., Jin, J., Frye, S. V., Earp, H. S., Graves, L. M., and Johnson, G. L. (2012) Dynamic repro-gramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer, Cell, 149, 307–321, doi: https://doi.org/10.1016/j.cell.2012.02.053.
Karoulia, Z., Wu, Y, Ahmed, T. A., Xin, Q., Bollard, J., Krepler, C., Wu, X., Zhang, C., Bollag, G., Herlyn, M., Fagin, J. A., Lujambio, A., Gavathiotis, E., and Poulikakos, P. I. (2016) An integrated model of RAF inhibitor action predicts inhibitor activity against oncogenic BRAF signaling, Cancer Cell, 30, 485–498, doi: https://doi.org/10.1016/j.ccell.2016.06.024.
Dardaei, L., Wang, H. Q., Singh, M., Fordjour, P., Shaw, K. X., et al. (2018) SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors, Nat. Med., 24, 512–517, doi: https://doi.org/10.1038/nm.4497.
Mainardi, S., Mulero-Sanchez, A., Prahallad, A., Germano, G., Bosma, A., Krimpenfort, P., Lieftink, C., Steinberg, J. D., de Wit, N., Goncalves-Ribeiro, S., Nadal, E., Bardelli, A., Villanueva, A., and Bernards, R. (2018) SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo, Nat. Med., 24, 961–967, doi: https://doi.org/10.1038/s41591-018-0023-9.
Funding
This study was supported by the Russian Foundation for Basic Research (project no. 18-315-00171 mol_a) and the Russian Science Foundation (grant no. 16-15-10423; construction of the BRAF V600E-expressing lentiviral vector and data presented in Fig. 1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethical standards
The article does not contain description of experiments with the participation of humans or animals performed by any of the authors.
Russian Text © The Author(s), 2020, published in Biokhimiya, 2020, Vol. 85, No. 1, pp. 126–138.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM19-271, December 2, 2019.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Putlyaeva, L.V., Demin, D.E., Uvarova, A.N. et al. PTPN11 Knockdown Prevents Changes in the Expression of Genes Controlling Cell Cycle, Chemotherapy Resistance, and Oncogene-Induced Senescence in Human Thyroid Cells Overexpressing BRAF V600E Oncogenic Protein. Biochemistry Moscow 85, 108–118 (2020). https://doi.org/10.1134/S0006297920010101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920010101