Abstract
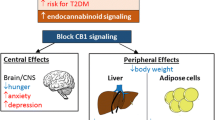
A growing number of evidences accumulated about critical metabolic role of cannabinoid type 1 receptor (CB1), carnitine palmitoyltransferase-1 (CPT1) and peroxisome proliferator-activated receptors (PPARs) in some peripheral tissues, including adipose tissue, liver, skeletal muscle and heart. To better understand the interactions of CB1, CPT1 and PPARs in these tissues, 30 diet-induced obese (DIO) C57BL/6J male mice were obtained, weight-matched and divided into two groups (15 in each group): (i) DIO/vehicle mice (D-Veh) and (ii) DIO/SR141716 mice (D-SR) treated with SR141716 (or rimonabant, a selective CB1 receptor blocker) administered orally (10 mg/kg daily). Another 15 mice fed standard diet (STD) formed the STD/vehicle group (S-Veh). At the end of 3-week treatment, mean body weight was 28.4 ± 0.5, 36.5 ± 0.8, and 30.3 ± 1.2 g for the S-Veh, D-Veh, and D-SR group, respectively (p < 0.05; D-Veh vs. D-SR). Liver weight in the D-SR group was also decreased significantly compared to the D-Veh group (p < 0.05). Serum levels of total cholesterol, high-density lipoprotein cholesterol, leptin and adiponectin in the D-SR group were ameliorated compared to the D-Veh group (p < 0.05). Both qRT-PCR and Western blot assay revealed that CB1 expression levels were efficiently blocked by SR141716 in subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), skeletal muscles and liver (D-SR vs. D-Veh; p < 0.05), whereas there was no significant difference between S-Veh and D-Veh mice (p > 0.05). Simultaneously with the reduction of CB1 expression in the D-SR group, the expression levels of CPT1A isoform (protein) in the liver and heart and CPT1B isoform (protein) in the SAT, VAT, liver and skeletal muscles were significantly increased (p < 0.05; D-SR vs. D-Veh). Interestingly, the CPT1A and CPT1B expression levels in heart were detected slightly. The expression levels of PPARα in the SAT, VAT, liver and skeletal muscles and PPARγ in the SAT and skeletal muscles in the D-SR group were significantly increased compared to the D-Veh mice (p < 0.05). However, the PPARβ expression level differed from that of PPARα and PPARγ. Taken together, these data indicate that the inhibition of CB1 could ameliorate lipid metabolism via the stimulation of the CPT1A and CPT1B expression in vivo. Simultaneously, the PPARα and PPARγ expression levels significantly differed compared to that of PPARβ in obesity and lipid metabolism-related disorders under blockade of CB1. Both the mechanism of the influence of CB1 inhibition on lipid metabolism in the examined tissues and the specific mechanism of PPARα, PPARγ and PPARβ involvement in lipid exchange under these conditions remain to be further elucidated.
Similar content being viewed by others

Abbreviations
- CB1:
-
cannabinoid type 1 receptor
- CPT1:
-
carnitine palmitoyltransferase 1
- DIO:
-
diet-induced obese (mice)
- HDL-C:
-
high-density lipoprotein cholesterol
- LDL-C:
-
low-density lipoprotein cholesterol
- PPARs:
-
peroxisome proliferator-activated receptors
- SAT:
-
subcutaneous adipose tissue
- STD:
-
standard diet
- TC:
-
total cholesterol
- TG:
-
triglycerides
- VAT:
-
visceral adipose tissue
References
Matias, I., Gonthier, M. P., Orlando, P., Martiadis, V., De, P. L., Cervino, C., Petrosino, S., Hoareau, L., Festy, F., and Pasquali, R. (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia, J. Clin. Endocr. Metab., 91, 3171–3180.
Mehrpouyabahrami, P., Chitrala, K. N., Ganewatta, M. S., Tang, C., Murphy, E. A., Enos, R. T., Velazquez, K. T., Mccellan, J., Nagarkatti, M., and Nagarkatti, P. (2017) Blockade of CB1 cannabinoid receptor alters gut microbio-ta and attenuates inflammation and diet-induced obesity, Sci. Rep., 7, 1–16.
Louet, J. F., Le May, C., Pegorier, J. P., Decaux, J. F., and Girard, J. (2001) Regulation of liver carnitine palmitoyl-transferase I gene expression by hormones and fatty acids, Biochem. Soc. Trans., 29, 310–316.
Musso, G., Gambino, R., and Cassader, M. (2009) Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD), Prog. Lipid Res., 48, 1–26.
Xu, H., Luo, J., Ma, G., Zhang, X., Yao, D., Li, M., and Loor, J. J. (2018) Acyl-CoA synthetase short-chain family member 2 (ACSS2) is regulated by SREBP-1 and plays a role in fatty acid synthesis in caprine mammary epithelial cells, J. Cell. Physiol., 233, 1005–1016.
Amengual, J., Petrov, P., Bonet, M. L., Ribot, J., and Palou, A. (2012) Induction of carnitine palmitoyl transferase 1 and fatty acid oxidation by retinoic acid in HepG2 cells, Int. J. Biochem. Cell B, 44, 2019–2027.
He, L., Kim, T., Long, Q., Liu, J., Wang, P., Zhou, Y., Ding, Y., Prasain, J., Wood, P. A., and Yang, Q. (2012) Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity, Circulation, 126, 1705–1716.
Ratner, C., Madsen, A. N., Kristensen, L. V., Skov, L. J., Pedersen, K. S., Mortensen, O. H., Knudsen, G. M., Raun, K., and Holst, B. (2015) Impaired oxidative capaci-ty due to decreased CPT1b levels as a contributing factor to fat accumulation in obesity, Am. J. Physiol. Regul. Integr. Comp. Physiol., 308, R973–R982.
Braissant, O., Foufelle, F., Scotto, C., Dauca, M., and Wahli, W. (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribu-tion of PPAR-alpha, -beta, and -gamma in the adult rat, Endocrinology, 137, 354–366.
O’Sullivan, S. E. (2007) Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors, Brit. J. Pharmacol., 152, 576–582.
Liu, L. Y., Alexa, K., Cortes, M., Schatzman-Bone, S., Kim, A. J., Mukhopadhyay, B., Cinar, R., Kunos, G., North, T. E., and Goessling, W. (2016) Cannabinoid receptor signaling regulates liver development and metabolism, Development, 143, 609–622.
Sun, Y., Alexander, S. P., Garle, M. J., Gibson, C. L., Hewitt, K., Murphy, S. P., Kendall, D. A., and Bennett, A. J. (2007) Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism, Brit. J. Pharmacol., 152, 734–743.
Palomer, X., Barroso, E., Zarei, M., Botteri, G., and Vazquez-Carrera, M. (2016) PPARβ/δ and lipid metabolism in the heart, Biochim. Biophys. Acta, 1861, 1569–1578.
Lehrke, M., and Lazar, M. A. (2006) The many faces of PPARgamma, Cell, 123, 993–999.
Duan, S. Z., Usher, M. G., and Mortensen, R. M. (2008) Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature, Circ. Res., 102, 283–294.
Zhang, Y. F., Yuan, Z. Q., Song, D. G., Zhou, X. H., and Wang, Y. Z. (2013) Effects of cannabinoid receptor 1 (brain) on lipid accumulation by transcriptional control of CPT1A and CPT1B, Anim. Genet., 45, 38–47.
Trillou, C. R., Arnone, M., Delgorge, C., Gonalons, N., Keane, P., Maffrand, J., and Soubrie, P. (2003) Anti-obesi-ty effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice, Am. J. Physiol. Regul. Integr. Comp. Physiol., 284, R345–R353.
Tam, J., Godlewski, G., Earley, B. J., Zhou, L., Jourdan, T., Szanda, G., Cinar, R., and Kunos, G. (2014) Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet-induced obesity, Am. J. Physiol. Endocr. Metab., 306, E457–E468.
Fu, L., Wei, L. W., Zhao, M. D., Zhu, J. L., Chen, S. Y., Jia, X. B., and Lai, S. J. (2016) Investigation of JAKs/STAT-3 in lipopolysaccharide-induced intestinal epithelial cells, Clin. Exp. Immunol., 186, 75–85.
Livak, K. J., and Schmittgen, T. D. (2001) Analysis of rela-tive gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method, Methods, 25, 402–408.
Katona, I., and Freund, T. F. (2012) Multiple functions of endocannabinoid signaling in the brain, Annu. Rev. Neurosci., 35, 529–558.
Lutz, B., Marsicano, G., Maldonado, R., and Hillard, C. J. (2015) The endocannabinoid system in guarding against fear, anxiety and stress, Nat. Rev. Neurosci., 16, 705–718.
Lu, H. C., and Mackie, K. (2016) An introduction to the endogenous cannabinoid system, Biol. Psychiat., 79, 516–525.
Rinaldi-Carmona, M., Barth, F., Heaulme, M., Shire, D., Calandra, B., Congy, C., Martinez, S., Maruani, J., Neliat, G., and Caput, D. (1994) SR141716A, a potent and selec-tive antagonist of the brain cannabinoid receptor, FEBS Lett., 350, 240–244.
Tang, Y., Ho, G., Li, Y., Hall, M. A., Hills, R. L., Black, S. C., Liang, Y., and Demarest, K. T. (2012) Beneficial metabolic effects of CB1R anti-sense oligonucleotide treatment in diet-induced obese AKR/J mice, PLOS One, 7, e42134.
Leite, C. E., Mocelin, C. A., Petersen, G. O., Leal, M. B., and Thiesen, F. V. (2009) Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity, Pharmacol. Rep. Pr., 61, 217–224.
Saxena, S., Thimmaraju, K. V., Srivastava, P. C., Mallick, A. K., Das, B., Sinha, N., and Dalmia, K. (2015) Role of dyslipidaemia and lipid peroxidation in pregnancy induced hypertension, J. Clin. Sci. Res., 4, 205–212.
Silvestri, C., Ligresti, A., and Marzo, V. D. (2011) Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle, Rev. Endocr. Metab. Dis., 12, 153–162.
Maslov, L. N., and Karpov, R. S. (2017) Prospects for the use of cannabinoid receptor ligands for the treatment of metabolic syndrome and atherosclerosis: analysis of exper-imental and clinical data, Vestn. Ross. Akad. Med. Nauk, 72, 59–65.
Nogueiras, R., Veyratdurebex, C., Suchanek, P. M., Klein, M., Wittmann, G., Watanabe, M., and Liposits, Z. (2008) Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats, Diabetes, 57, 2977–2991.
Osei-Hyiaman, D., Liu, J. L., Godlewski, G., Harvey-White, J., Jeong, W., Batkai, S., Marsicano, G., Lutz, B., Buettner, C., and Kunos, G. (2008) Hepatic CB1 receptor is required for development of diet-induced steatosis, dys-lipidemia, and insulin and leptin resistance in mice, J. Clin. Invest., 118, 3160–3169.
Veiga, F., Graus-Nunes, F., Rachid, T. L., Barreto, A. B., Mandarim-de-Lacerda, C. A., and Souza-Mello, V. (2017) Anti-obesogenic effects of WY14643 (PPAR-alpha ago-nist): hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice, Biochimie, 140, 106–116.
Pagano, C., Pilon, C., Calcagno, A., Urbanet, R., Rossato, M., Milan, G., Bianchi, K., Rizzuto, R., Bernante, P., and Federspil, G. (2007) The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms, J. Clin. Endocr. Metab., 92, 4810–4819.
Wang, S., Dougherty, E. J., and Danner, R. L. (2016) PPARγ signaling and emerging opportunities for improved therapeutics, Pharmacol. Res., 111, 76–85.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, L.W., Yuan, Z.Q., Zhao, M.D. et al. Inhibition of Cannabinoid Receptor 1 Can Influence the Lipid Metabolism in Mice with Diet-Induced Obesity. Biochemistry Moscow 83, 1279–1287 (2018). https://doi.org/10.1134/S0006297918100127
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918100127