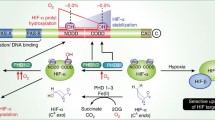
Abstract
An organism naturally responds to hypoxia via stabilization of hypoxia-inducible factor (HIF). There are three isoforms of HIFα subunits whose stability is regulated by three isozymes of HIF prolyl hydroxylase (PHD1-3). Despite intense studies on recombinant enzyme isoforms using homogeneous activity assay, there is no consensus on the PHD iso-form preference for the HIF isoform as a substrate. This work provides a new approach to the problem of substrate specificity using cell-based reporters expressing the enzyme and luciferase-labeled substrate pair encoded in the same expression vector. The cell is used as a microbioreactor for running the reaction between the overexpressed enzyme and substrate. Using this novel approach, no PHD3 activity toward HIF3 was demonstrated, indirectly pointing to the hydroxylation of the second proline in 564PYIP567 (HIF1) catalyzed by this isozyme. The use of “paired” enzyme–substrate reporters to evaluate the potency of “branched tail” oxyquinoline inhibitors of HIF PHD allows higher precision in revealing the optimal structural motif for each enzyme isoform.
Similar content being viewed by others
Abbreviations
- αKG:
-
alpha-ketoglutarate
- DMSO:
-
dimethylsulf-oxide
- HIF:
-
hypoxia-inducible factor
- ODD:
-
oxygen-dependent degradation
- PHD:
-
prolyl hydroxylase domain
- VHL:
-
von Hippel–Lindau protein
References
Ivan, M., and Kaelin, W. G., Jr. (2017) The EGLN-HIF O2-sensing system: multiple inputs and feedbacks, Mol. Cell, 66, 772–779.
Koivunen, P., Tiainen, P., Hyvarinen, J., Williams, K. E., Sormunen, R., Klaus, S. J., Kivirikko, K. I., and Myllyharju, J. (2007) An endoplasmic reticulum transmembrane prolyl 4-hydroxylase is induced by hypoxia and acts on hypoxia-inducible factor alpha, J. Biol. Chem., 282, 30544–30552.
Xie, L., Xiao, K., Whalen, E. J., Forrester, M. T., Freeman, R. S., Fong, G., Gygi, S. P., Lefkowitz, R. J., and Stamler, J. S. (2009) Oxygen-regulated beta(2)-adrenergic receptor hydroxylation by EGLN3 and ubiquitylation by pVHL, Sci. Signal., 2, ra33.
Xie, L., Pi, X., Mishra, A., Fong, G., Peng, J., and Patterson, C. (2009) PHD3-dependent hydroxylation of HCLK2 promotes the DNA damage response, J. Clin. Invest., 122, 2827–2836.
Luo, W., Lin, B., Wang, Y., Zhong, J., O’Meally, R., Cole, R. N., Pandey, A., Levchenko, A., and Semenza, G. L. (2014) PHD3-mediated prolyl hydroxylation of nonmuscle actin impairs polymerization and cell motility, Mol. Biol. Cell, 25, 2788–2796.
Heir, P., Srikumar, T., Bikopoulos, G., Bunda, S., Poon, B. P., Lee, J. E., Raught, B., and Ohh, M. (2016) Oxygen-dependent regulation of erythropoietin receptor turnover and signaling, J. Biol. Chem., 291, 7357–7372.
German, N. J., Yoon, H., Yusuf, R. Z., Murphy, J. P., Finley, L. W., Laurent, G., Haas, W., Satterstrom, F. K., Guarnerio, J., Zaganjor, E., Santos, D., Pandolfi, P. P., Beck, A. H., Gygi, S. P., Scadden, D. T., Kaelin, W. G., Jr., and Haigis, M. C. (2016) PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2, Mol. Cell, 63, 1006–1020.
Pappalardi, M. B., McN Su, Y., Ming, G. L., Song, H., Cave, J. W., Schofield, C. J., Colbourne, F., Coppola, G., and Ratan, R. R. (2016) Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates ATF4-dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models, Sci. Transl. Med., 8, 328ra29.
Murray, J. K., Balan, C., Allgeier, A. M., Kasparian, A., Viswanadhan, V., Wilde, C., Allen, J. R., Yoder, S. C., Biddlecome, G., Hungate, R. W., and Miranda, L. P. (2010) Dipeptidyl-quinolone derivatives inhibit hypoxia inducible factor-1α prolyl hydroxylases -1, -2, and -3 with altered selectivity, J. Combin. Chem., 12, 676–686.
Siddiq, A., Aminova, L. R., Troy, C. M., Suh, K., Messer, Z., Semenza, G. L., and Ratan, R. R. (2009) Selective inhibition of hypoxia-inducible factor prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF and CREB independent pathways, J. Neurosci., 29, 8828–8838.
Fedulova, N., Hanrieder, J., Bergquist, J., and Emren, L. O. (2007) Expression and purification of catalytically active human PHD3 in Escherichia coli, Protein Express. Purif., 54, 1–10.
Minervini, G., Masiero, A., Moro, S., and Tosatto, S. C. (2013) In silico investigation of PHD-3 specific HIF1-α proline 567 hydroxylation: a new player in the VHL/HIF-1α interaction pathway? FEBS Lett., 587, 2996–3001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM17-394, September 18, 2017.
Rights and permissions
About this article
Cite this article
Osipyants, A.I., Smirnova, N.A., Khristichenko, A.Y. et al. Enzyme–substrate reporters for evaluation of substrate specificity of HIF prolyl hydroxylase isoforms. Biochemistry Moscow 82, 1207–1214 (2017). https://doi.org/10.1134/S0006297917100145
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917100145