Abstract
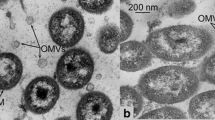
The Gram-negative bacterium Lysobacter sp. XL1 produces outer membrane vesicles that are heterogeneous in size, density, and protein composition. One of the subpopulations is secretory vesicles for lytic protease L5 of Lysobacter sp. XL1 (Kudryakova et al. (2015) FEMS Microbiol. Lett., 362, fnv137). Protein L5 was assumed to influence biogenesis of these secretory vesicles that contain it. Using a Pseudomonas fluorescens Q2-87/B expression system, it was shown that the recombinant L5 protein may act as a factor of vesicle biogenesis. This points to a possible involvement of L5 protein in Lysobacter sp. XL1 vesicle biogenesis. Furthermore, it was established that the main phospholipid of Lysobacter sp. XL1 vesicles is cardiolipin, and vesicles are formed predominantly of outer membrane regions enriched with this phospholipid. This indicates that cardiolipin participates in biogenesis of all vesicle subpopulations in Lysobacter sp. XL1.
Similar content being viewed by others
References
Chatterjee, S. N., and Das, J. (1967) Electron microscopic observations on the excretion of cell-wall material by Vibrio cholera, J. Gen. Microbiol., 49, 1–11.
Mayrand, D., and Grenier, D. (1989) Biological activities of outer membrane vesicles, Can. J. Microbiol., 35, 607–613.
Kuehn, M. J., and Kesty, N. C. (2005) Bacterial outer membrane vesicles and the host–pathogen interaction, Genes Dev., 19, 2645–2655.
Amano, A., Takeuchi, H., and Furuta, N. (2010) Outer membrane vesicles function as offensive weapons in host–parasite interactions, Microbes Infect., 12, 791–798.
Tashiro, Y., Uchiyama, H., and Nomura, N. (2012) Multifunctional membrane vesicles in Pseudomonas aeruginosa, Environ. Microbiol., 14, 1349–1362.
Kulkarni, H. M., and Jagannadham, M. V. (2014) Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria, Microbiology, 160, 2109–2121.
Avila-Calderon, E. D., Araiza-Villanueva, M. G., CancinoDiaz, J. C., Lopez-Villegas, E. O., Sriranganathan, N., Boyle, S. M., and Contreras-Rodriguez, A. (2015) Roles of bacterial membrane vesicles, Arch. Microbiol., 197, 1–10.
Kadurugamuwa, J. L., and Beveridge, T. J. (1995) Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion, J. Bacteriol., 177, 3998–4008.
Horstman, A. L., and Kuehn, M. J. (2000) Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles, J. Biol. Chem., 275, 12489–12496.
Roier, S., Blume, T., Klug, L., Wagner, G. E., Elhenawy, W., Zangger, K., Prassl, R., Reidl, J., Daum, G., Feldman, M. F., and Schild, S. (2015) A basis for vaccine development: comparative characterization of Haemophilus influenzae outer membrane vesicles, Int. J. Med. Microbiol., 305, 298–309.
Olofsson, A., Vallstrom, A., Petzold, K., Tegtmeyer, N., Schleucher, J., Carlsson, S., Haas, R., Backert, S., Wai, S. N., Grobner, G., and Arnqvist, A. (2010) Biochemical and functional characterization of Helicobacter pylori vesicles, Mol. Microbiol., 77, 1539–1555.
Perez-Cruz, C., Delgado, L., Lopez-Iglesias, C., and Mercade, E. (2015) Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria, PLoS One, 10, e0116896.
Lee, E. Y., Choi, D. S., Kim, K. P., and Gho, Y. S. (2008) Proteomics in gram-negative bacterial outer membrane vesicles, Mass Spectrom. Rev., 27, 535–555.
Li, Z., Clarke, A. J., and Beveridge, T. J. (1996) A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles, J. Bacteriol., 178, 2479–2488.
Ciofu, O., Beveridge, T. J., Kadurugamuwa, J., WaltherRasmußsen, J., and Hoiby, N. (2000) Chromosomal ß-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa, J. Antimicrob. Chemother., 45, 9–13.
Kobayashi, H., Uematsu, K., Hirayama, H., and Horikoshi, K. (2000) Novel toluene elimination system in a toluenetolerant microorganism, J. Bacteriol., 182, 6451–6455.
Kunsmann, L., Ruter, C., Bauwens, A., Greune, L., Gluder, M., Kemper, B., Fruth, A., Wai, S. N., He, X., Lloubes, R., Schmidt, M. A., Dobrindt, U., Mellmann, A., Karch, H., and Bielaszewska, M. (2015) Virulence from vesicles: novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain, Sci. Rep., 5, 13252.
Olsen, I., and Amano, A. (2015) Outer membrane vesicles–offensive weapons or good Samaritans? J. Oral Microbiol., 7, 27468.
Gujrati, V., Kim, S., Kim, S. H., Min, J. J., Choy, H. E., Kim, S. C., and Jon, S. (2014) Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy, ACS Nano, 8, 1525–1537.
Mashburn-Warren, L. M., and Whiteley, M. (2006) Special delivery: vesicle trafficking in prokaryotes, Mol. Microbiol., 61, 839–846.
Kulp, A., and Kuehn, M. J. (2010) Biological functions and biogenesis of secreted bacterial outer membrane vesicles, Annu. Rev. Microbiol., 64, 163–184.
Schwechheimer, C., Kulp, A., and Kuehn, M. J. (2014) Modulation of bacterial outer membrane vesicle production by envelope structure and content, BMC Microbiol., 14, 324.
Schwechheimer, C., and Kuehn, M. J. (2015) Outer membrane vesicles from Gram-negative bacteria: biogenesis and functions, Nat. Rev. Microbiol., 13, 605–619.
Hoekstra, D., Van der Laan, J. W., De Leij, L., and Witholt, B. (1976) Release of outer membrane fragments from normally growing Escherichia coli, Biochim. Biophys. Acta, 455, 889–899.
Wensink, J., and Witholt, B. (1981) Outer membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein, Eur. J. Biochem., 116, 331–335.
Schertzer, J. W., and Whiteley, M. (2012) A bilayer-couple model of bacterial outer membrane vesicle biogenesis, MBio, 3, e00297–11.
Zhou, L., Srisatjaluk, R., Justus, D. E., and Doyle, R. J. (1998) On the origin of membrane vesicles in gram-negative bacteria, FEMS Microbiol. Lett., 163, 223–228.
Hayashi, J., Hamada, N., and Kuramitsu, H. K. (2002) The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release, FEMS Microbiol. Lett., 216, 217–222.
Balsalobre, C., Silvan, J. M., Berglund, S., Mizunoe, Y., Uhlin, B. E., and Wai, S. N. (2006) Release of the type I secreted a-haemolysin via outer membrane vesicles from Escherichia coli, Mol. Microbiol., 59, 99–112.
Rompikuntal, P. K., Thay, B., Khan, M. K., Alanko, J., Penttinen, A. M., Asikainen, S., Wai, S. N., and Oscarsson, J. (2012) Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans, Infect. Immun., 80, 31–42.
Stepnaia, O. A., Severin, A. I., Kudryavtseva, A. I., Krupyanko, V. I., Kozlovsky, A. G., and Kulaev, I. S. (1992) Enzymes of bacteriolytic preparation lysoamidase. Some properties of bacteriolytic proteinase L2, Prikl. Biokhim. Mikrobiol., 28, 666–673.
Stepnaya, O. A., Begunova, E. A., Tsfasman, I. M., and Kulaev, I. S. (1996) Bacteriolytic enzyme preparation lysoamidase: isolation and some physicochemical properties of extracellular muramidase from Xanthomonas sp. bacteria, Biochemistry (Moscow), 61, 471–476.
Muranova, T. A., Krasovskaya, L. A., Tsfasman, I. M., Stepnaya, O. A., and Kulaev, I. S. (2004) Structural investigations and identification of the extracellular bacteriolytic endopeptidase L1 from Lysobacter sp., Biochemistry (Moscow), 69, 501–505.
Stepnaya, O. A., Tsfasman, I. M., Logvina, I. A., Ryazanova, L. P., Muranova, T. A., and Kulaev, I. S. (2005) Isolation and characterization of a new extracellular bacteriolytic endopeptidase of Lysobacter sp. XL1, Biochemistry (Moscow), 70, 1031–1037.
Vasilyeva, N. V., Tsfasman, I. M., Suzina, N. E., Stepnaya, O. A., and Kulaev, I. S. (2008) Secretion of bacteriolytic endopeptidase L5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles, FEBS J., 275, 3827–3835.
Vasilyeva, N. V., Shishkova, N. A., Marinin, L. I., Ledova, L. A., Tsfasman, I. M., Muranova, T. A., Stepnaya, O. A., and Kulaev, I. S. (2014) Lytic peptidase L5 of Lysobacter sp. XL1 with broad antimicrobial spectrum, J. Mol. Microbiol. Biotechnol., 24, 59–66.
Granovsky, I. E., Kalinin, A. E., Lapteva, Y. S., Latypov, O. R., Vasilyeva, N. V., Tsfasman, I. M., Stepnaya, O. A., Kulaev, I. S., Muranova, T. A., and Krasovskaia, L. A. (2011) Lytic Enzyme AlpB of Bacteria Lysobacter sp. XLI, DNA Fragment Encoding the Lytic Enzyme AlpB of Bacteria Lysobacter sp. XLI, and Purification of the Lytic Enzyme AlpB of Bacteria Lysobacter sp. XLI, RFPatent No. 2408725 [in Russian].
Kudryakova, I. V., Suzina, N. E., and Vasilyeva, N. V. (2015) Biogenesis of Lysobacter sp. XL1 vesicles, FEMS Microbiol. Lett., 362, fnv137.
Lapteva, Y. S., Zolova, O. E., Shlyapnikov, M. G., Tsfasman, I. M., Muranova, T. A., Stepnaya, O. A., Kulaev, I. S., and Granovsky, I. E. (2012) Cloning and expression analysis of genes encoding lytic endopeptidases L1 and L5 from Lysobacter sp. strain XL1, Appl. Environ. Microbiol., 78, 7082–7089.
Tsfasman, I. M., Lapteva, Y. S., Krasovskaya, L. A., Kudryakova, I. V., Vasilyeva, N. V., Granovsky, I. E., and Stepnaya, O. A. (2015) Gene expression of lytic endopeptidases AlpA and AlpB from Lysobacter sp. XL1 in Pseudomonads, J. Mol. Microbiol. Biotechnol., 25, 244–252.
Grenier, D., and Mayrand, D. (1987) Functional characterization of extracellular vesicles produced by Bacteroides gingivalis, Infect. Immun., 55, 111–117.
Osborn, M. J., Gander, J. E., Parisi, E., and Carson, J. (1972) Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane, J. Biol. Chem., 247, 3962–3972.
Ames, G. F. (1968) Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism, J. Bacteriol., 95, 833–843.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) Protein measurement with the Folin phenol reagent, J. Biol. Chem., 193, 265–275.
Karkhanis, Y. D., Zeltner, J. Y., Jackson, J. J., and Carlo, D. J. (1978) A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gramnegative bacteria, Anal. Biochem., 85, 595–601.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 227, 680–685.
Choi, D. S., Kim, D. K., Choi, S. J., Lee, J., Choi, J. P., Rho, S., Park, S. H., Kim, Y. K., Hwang, D., and Gho, Y. S. (2011) Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa, Proteomics, 11, 3424–3429.
Bauman, S. J., and Kuehn, M. J. (2006) Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response, Microbes Infect., 8, 2400–2408.
Tashiro, Y., Ichikawa, S., Shimizu, M., Toyofuku, M., Takaya, N., Nakajima-Kambe, T., Uchiyama, H., and Nomura, N. (2010) Variation of physiochemical properties and cell association activity of membrane vesicles with growth phase in Pseudomonas aeruginosa, Appl. Environ. Microbiol., 76, 3732–3739.
Lappann, M., Otto, A., Becher, D., and Vogel, U. (2013) Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitides, J. Bacteriol., 195, 4425–4435.
Chowdhury, C., and Jagannadham, M. V. (2013) Virulence factors are released in association with outer membrane vesicles of Pseudomonas syringae pv. tomato T1 during normal growth, Biochim. Biophys. Acta, 1834, 231–239.
Kulkarni, H. M., Swamy, Ch. V., and Jagannadham, M. V. (2014) Molecular characterization and functional analysis of outer membrane vesicles from the Antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions, J. Proteome Res., 13, 1345–1358.
Tashiro, Y., Inagaki, A., Shimizu, M., Ichikawa, S., Takaya, N., Nakajima-Kambe, T., Uchiyama, H., and Nomura, N. (2011) Characterization of phospholipids in membrane vesicles derived from Pseudomonas aeruginosa, Biosci. Biotechnol. Biochem., 75, 605–607.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I. V. Kudryakova, N. E. Suzina, N. G. Vinokurova, N. A. Shishkova, N. V. Vasilyeva, 2017, published in Biokhimiya, 2017, Vol. 82, No. 4, pp. 677-686.
Rights and permissions
About this article
Cite this article
Kudryakova, I.V., Suzina, N.E., Vinokurova, N.G. et al. Studying factors involved in biogenesis of Lysobacter sp. XL1 outer membrane vesicles. Biochemistry Moscow 82, 501–509 (2017). https://doi.org/10.1134/S0006297917040125
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917040125