Abstract
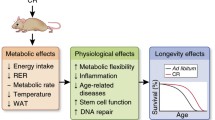
Food restriction causes a set of physiological changes that reduce the rate of aging. At the level of an organism, these changes are initiated by a hormonal response, which in turn activates certain intracellular signaling cascades. As a result, cells increase their antioxidant capacities and decrease the risk of cancerous transformation. A number of small molecule compounds activating these signaling cascades have been described. One could expect that direct pharmacological activation of the signaling can produce a stronger antiaging effect than that achieved by the indirect hormonal stimulation. Data from the literature point to the opposite. Possibly, a problem with pharmacological activators is that they cause generation of mitochondrial reactive oxygen species. Indeed, hyperpolarized mitochondria are known to induce oxidative stress. Such hyperpolarization could happen because of artificial activation of cellular response to caloric restriction in the absence of energy deficit. At the same time, energy deficit seems likely to be a natural consequence of the shortage of nutrients. Thus, there is a possibility that combining the pharmacological activators with compounds that decrease mitochondrial transmembrane potential, uncouplers, could be a powerful antiaging strategy.
Similar content being viewed by others
Abbreviations
- AICAR:
-
5-aminoimidazole-4-carboxamide ribonucleoside
- AMPK:
-
AMP-dependent protein kinase
- CR:
-
caloric restriction
- C12TPP:
-
dodecyltriphenylphosphonium
- FCCP:
-
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- GH:
-
growth hormone (somatotropin)
- IGF1:
-
insulin-like growth factor 1
- ROS:
-
reactive oxygen species
References
Spindler, S. R. (2010) Caloric restriction: from soup to nuts, Ageing Res. Rev., 9, 324–353.
Ruiz, R., Pedrez-Villegas, E. M., and Manuel Carrion, A. (2016) AMPK function in aging process, Curr. Drug Targets, 17, 932–941.
Burkewitz, K., Zhang, Y., and Mair, W. B. (2014) AMPK at the nexus of energetics and aging, Cell Metab., 20, 10–25.
Verdin, E. (2015) NAD+ in aging, metabolism, and neurodegeneration, Science, 350, 1208–1213.
Gremeaux, V., Gayda, M., Lepers, R., Sosner, P., Juneau, M., and Nigam, A. (2012) Exercise and longevity, Maturitas, 73, 312–317.
Lagisz, M., Hector, K. L., and Nakagawa, S. (2013) Life extension after heat shock exposure: assessing meta-analytic evidence for hormesis, Ageing Res. Rev., 12, 653–660.
Munkacsy, E., and Rea, S. L. (2014) The paradox of mitochondrial dysfunction and extended longevity, Exp. Gerontol., 56, 221–233.
Ristow, M., and Zarse, K. (2010) How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis), Exp. Gerontol., 45, 410–418.
Sanli, T., Steinberg, G. R., Singh, G., and Tsakiridis, T. (2014) AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway, Cancer Biol. Ther., 15, 156–169.
Kahn, B. B., Alquier, T., Carling, D., and Hardie, D. G. (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism, Cell Metab., 1, 15–25.
Caldeira da Silva, C. C., Cerqueira, F. M., Barbosa, L. F., Medeiros, M. H. G., and Kowaltowski, A. J. (2008) Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity, Aging Cell, 7, 552–560.
Ruderman, N. B., Xu, X. J., Nelson, L., Cacicedo, J. M., Saha, A. K., Lan, F., and Ido, Y. (2010) AMPK and SIRT1: a long-standing partnership? Am. J. Physiol., 298, 137–163.
Ingram, D. K., and Roth, G. S. (2015) Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res. Rev., 20, 46–62.
Mercken, E. M., Carboneau, B. A., Krzysik-Walker, S. M., and De Cabo, R. (2012) Of mice and men: the benefits of caloric restriction, exercise, and mimetics, Ageing Res. Rev., 11, 390–398.
Testa, G., Biasi, F., Poli, G., and Chiarpotto, E. (2014) Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity, Curr. Pharm. Design., 20, 2950–2977.
Green, D. R., Galluzzi, L., and Kroemer, G. (2014) Cell biology. Metabolic control of cell death, Science, 345, 1250256.
Seifert, E. L., Bezaire, V., Estey, C., and Harper, M.-E. (2008) Essential role for uncoupling protein-3 in mitochondrial adaptation to fasting but not in fatty acid oxidation or fatty acid anion export, J. Biol. Chem., 283, 25124–25131.
Wang, C.-M., Almsherqi, Z. A., McLachlan, C. S., Matthews, S., Ramachandran, M., Tay, S. K. H., and Deng, Y. (2011) Acute starvation in C57BL/6J mice increases myocardial UCP2 and UCP3 protein expression levels and decreases mitochondrial bio-energetic function, Stress, 14, 66–72.
Suwa, M., Nakano, H., and Kumagai, S. (2003) Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles, J. Appl. Physiol., 95, 960–968.
Fedorenko, A., Lishko, P. V., and Kirichok, Y. (2012) Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria, Cell, 151, 400–413.
Sluse, F. E., Jarmuszkiewicz, W., Navet, R., Douette, P., Mathy, G., and Sluse-Goffart, C. M. (2006) Mitochondrial UCPs: new insights into regulation and impact, Biochim. Biophys. Acta, 1757, 480–485.
Garlid, K. D., Jaburek, M., and Jezek, P. (2001) Mechanism of uncoupling protein action, Biochem. Soc. Trans., 29, 803–806.
Korshunov, S. S., Skulachev, V. P., and Starkov, A. A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria, FEBS Lett., 416, 15–18.
Skulachev, V. P. (1996) Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants, Quart. Rev. Biophys., 29, 169–202.
Daval, M., Foufelle, F., and Ferre, P. (2006) Functions of AMP-activated protein kinase in adipose tissue, J. Physiol., 574, 55–62.
Atkinson, D. E. (1968) The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers, Biochemistry, 7, 4030–4034.
Klingenberg, M. (2008) The ADP and ATP transport in mitochondria and its carrier, Biochim. Biophys. Acta, 1778, 1978–2021.
Massudi, H., Grant, R., Guillemin, G. J., and Braidy, N. (2012) NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns, Redox Rep., 17, 28–46.
Hubbard, B. P., and Sinclair, D. A. (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases, Trends Pharmacol. Sci., 35, 146–154.
Weisova, P., Anilkumar, U., Ryan, C., Concannon, C. G., Prehn, J. H. M., and Ward, M. W. (2012) “Mild mitochondrial uncoupling” induced protection against neuronal excitotoxicity requires AMPK activity, Biochim. Biophys. Acta, 1817, 744–753.
Zorov, D. B. (1996) Mitochondrial damage as a source of diseases and aging: a strategy of how to fight these, Biochim. Biophys. Acta, 1275, 10–15.
Starkov, A. A. (1997) “Mild” uncoupling of mitochondria, Biosci. Rep., 17, 273–279.
Izyumov, D. S., Avetisyan, A. V., Pletjushkina, O. Y., Sakharov, D. V., Wirtz, K. W., Chernyak, B. V., and Skulachev, V. P. (2004) “Wages of Fear”: transient threefold decrease in intracellular ATP level imposes apoptosis, Biochim. Biophys. Acta, 1658, 141–147.
Bodur, C., Karakas, B., Timucin, A. C., Tezil, T., and Basaga, H. (2015) AMP-activated protein kinase couples 3-bromopyruvate-induced energy depletion to apoptosis via activation of FoxO3a and upregulation of proapoptotic Bcl-2 proteins, Mol. Carcinog., doi: 10.1002/mc.22411.
Shin, S., Buel, G. R., Wolgamott, L., Plas, D. R., Asara, J. M., Blenis, J., and Yoon, S. O. (2015) ERK2 mediates metabolic stress response to regulate cell fate, Mol. Cell, 59, 382–398.
Meynet, O., Zunino, B., Happo, L., Pradelli, L. A., Chiche, J., Jacquin, M. A., Mondragon, L., Tanti, J. F., Taillan, B., Garnier, G., Reverso-Meinietti, J., Mounier, N., Michiels, J. F., Michalak. E. M., Carles, M., Scott, C. L., and Ricci, J. E. (2013) Caloric restriction modulates Mcl-1 expression and sensitizes lymphomas to BH3 mimetic in mice, Blood, 122, 2402–2411.
Tasyurek, H. M., Altunbas, H. A., Balci, M. K., and Sanlioglu, S. (2014) Incretins: their physiology and application in the treatment of diabetes mellitus, Diab. Metab. Res. Rev., 30, 354–371.
Tian, L., and Jin, T. (2016) The incretin hormone GLP-1 and mechanisms underlying its secretion, J. Diabetes, http://dx.doi.org/10.1111/1753-0407.12439.
Reimann, F., and Gribble, F. M. (2016) Mechanisms underlying glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 secretion, J. Diab. Invest., 7 (Suppl. 1), 13–19.
Svendsen, B., and Holst, J. J. (2016) Regulation of gut hormone secretion. Studies using isolated perfused intestines, Peptides, 77, 47–53.
Rorsman, P., and Braun, M. (2013) Regulation of insulin secretion in human pancreatic islets, Annu. Rev. Physiol., 75, 155–179.
Pais, R., Gribble, F. M., and Reimann, F. (2016) Stimulation of incretin secreting cells, Ther. Adv. Endocrinol. Metab., 7, 24–42.
Brown-Borg, H. M., and Bartke, A. (2012) GH and IGF1: roles in energy metabolism of long-living GH mutant mice, J. Gerontol. Ser. A Biol. Sci. Med. Sci., 67, 652–660.
Bartke, A., Sun, L. Y., and Longo, V. (2013) Somatotropic signaling: trade-offs between growth, reproductive development, and longevity, Physiol. Rev., 93, 571–598.
Esteban, S., Garau, C., Aparicio, S., Moranta, D., Barcelo, P., Ramis, M., Tresguerres, J. A., and Rial, R. (2010) Improving effects of long-term growth hormone treatment on monoaminergic neurotransmission and related behavioral tests in aged rats, Rejuv. Res., 13, 707–716.
Zouboulis, C. C., and Makrantonaki, E. (2012) Hormonal therapy of intrinsic aging, Rejuv. Res., 15, 302–312.
Paredes, S. D., Forman, K. A., Garcia, C., Vara, E., Escames, G., and Tresguerres, J. A. F. (2014) Protective actions of melatonin and growth hormone on the aged cardiovascular system, Horm. Mol. Biol. Clin. Invest., 18, 79–88.
Bartke, A., List, E. O., and Kopchick, J. J. (2016) The somatotropic axis and aging: benefits of endocrine defects, Growth Horm. Res., 27, 41–45.
Cheng, Z., Tseng, Y., and White, M. F. (2010) Insulin signaling meets mitochondria in metabolism, Trends Endocrinol. Metab., 21, 589–598.
Sharples, A. P., Hughes, D. C., Deane, C. S., Saini, A., Selman, C., and Stewart, C. E. (2015) Longevity and skeletal muscle mass: the role of IGF signaling, the sirtuins, dietary restriction and protein intake, Aging Cell, 14, 511–523.
Hindupur, S. K., Gonzalez, A., and Hall, M. N. (2015) The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control, Cold Spring Harb. Perspect. Biol., 7, a019141.
Blagosklonny, M. V. (2012) Prospective treatment of agerelated diseases by slowing down aging, Am. J. Pathol., 181, 1142–1146.
Xu, S., Cai, Y., and Wei, Y. (2014) mTOR signaling from cellular senescence to organismal aging, Aging Dis., 5, 263–273.
McLeod, M., Breen, L., Hamilton, D. L., and Philp, A. (2016) Live strong and prosper: the importance of skeletal muscle strength for healthy ageing, Biogerontology, 17, 497–510.
Russell, A. P., Foletta, V. C., Snow, R. J., and Wadley, G. D. (2014) Skeletal muscle mitochondria: a major player in exercise, health and disease, Biochim. Biophys. Acta, 1840, 1276–1284.
Vina, J., Sanchis-Gomar, F., Martinez-Bello, V., and Gomez-Cabrera, M. C. (2012) Exercise acts as a drug; the pharmacological benefits of exercise, Br. J. Pharmacol., 167, 1–12.
Harman, D. (1956) Aging: a theory based on free radical and radiation chemistry, J. Gerontol., 11, 298–300.
Knorre, D. A., and Severin, F. F. (2012) Longevity and mitochondrial membrane potential, Biochemistry (Moscow), 77, 793–794.
Kamour, A., George, N., Gwynnette, D., Cooper, G., Lupton, D., Eddleston, M., Thompson, J. P., Vale, J. A., Thanacoody, H. K., Hill, S., and Thomas, S. H. (2015) Increasing frequency of severe clinical toxicity after use of 2,4-dinitrophenol in the UK: a report from the National Poisons Information Service, Emerg. Med. J., 32, 383–386.
Grundlingh, J., Dargan, P. I., El-Zanfaly, M., and Wood, D. M. (2011) 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death, J. Med. Toxicol., 7, 205–212.
Severin, F. F., Severina, I. I., Antonenko, Y. N., Rokitskaya, T. I., Cherepanov, D. A., Mokhova, E. N., Vyssokikh, M. Y., Pustovidko, A. V., Markova, O. V., Yaguzhinsky, L. S., Korshunova, G. A., Sumbatyan, N. V., Skulachev, M. V., and Skulachev, V. P. (2010) Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore, Proc. Natl. Acad. Sci. USA, 107, 663–668.
Antonenko, Y. N., Avetisyan, A. V., Cherepanov, D. A., Knorre, D. A., Korshunova, G. A., Markova, O. V., Ojovan, S. M., Perevoshchikova, I. V., Pustovidko, A. V., Rokitskaya, T. I., Severina, I. I., Simonyan, R. A., Smirnova, E. A., Sobko, A. A., Sumbatyan, N. V., Severin, F. F., and Skulachev, V. P. (2011) Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers, J. Biol. Chem., 286, 17831–17840.
Kalinovich, A. V., Mattsson, C. L., Mohamed R. Youssef, M. R., Petrovic, N., Ost, M., Skulachev, V. P., and Shabalina, I. G. (2016) Mitochondria-targeted dodecyltriphenylphosphonium (C12TPP) combats high-fat 2 dietinduced obesity in mice, Int. J. Obesity, accepted manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2016, Vol. 81, No. 12, pp. 1713–1720.
Rights and permissions
About this article
Cite this article
Knorre, D.A., Severin, F.F. Uncouplers of oxidation and phosphorylation as antiaging compounds. Biochemistry Moscow 81, 1438–1444 (2016). https://doi.org/10.1134/S0006297916120051
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916120051