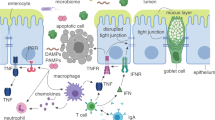
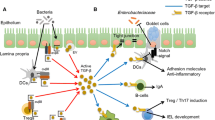
Abstract
Ulcerative colitis and Crohn’s disease are the major forms of inflammatory bowel disease. Cytokines of the tumor necrosis factor (TNF) family play an important role in the regulation of intestinal inflammation. In this review, we discuss the function of key cytokines of this family–TNF and lymphotoxin (LT)–in mucosal healing, IgA production, and in control of innate lymphoid cells (ILCs), novel regulators of mucosal homeostasis in the gut. TNF plays a central role in the pathogenesis of inflammatory bowel diseases (IBD). LT regulates group 3 of ILCs and IL-22 production and protects the epithelium against damage by chemicals and mucosal bacterial pathogens. In addition, we discuss major mouse models employed to study the mechanism of intestinal inflammation, their advantages and limitations, as well as application of TNF blockers in the therapy for IBD.
Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- DCs:
-
dendritic cells
- DSS:
-
dextran sulfate sodium
- FDA:
-
Food and Drug Administration of USA
- FDC:
-
follicular dendritic cells
- GALT:
-
gut-associated lymphoid tissue
- IBD:
-
inflammatory bowel disease
- IFN:
-
interferon
- IL:
-
interleukin
- ILCs:
-
innate lymphoid cells
- LIGHT:
-
homologous to lymphotoxin, exhibits inducible expression and competes with HSV glycoprotein D for binding to herpesvirus entry mediator, a receptor expressed on T-lymphocytes (another name: tumor necrosis factor superfamily member 14, TNFSF14)
- LT:
-
lymphotoxin
- LTßR:
-
membrane lymphotoxin receptor
- MHC:
-
major histocompatibility complex
- MNP:
-
mononuclear phagocytes
- TACE:
-
TNF-alpha converting enzyme
- TNBS:
-
2,4,6-trinitrobenzenesulfonic acid
- TNF:
-
tumor necrosis factor
- TNFR:
-
tumor necrosis factor receptor
- UC:
-
ulcerative colitis
References
Locksley, R. M., Killeen, N., and Lenardo, M. J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology, Cell, 104, 487–501.
Ward-Kavanagh, L. K., Lin, W. W., Sedy, J. R., and Ware, C. F. (2016) The TNF receptor superfamily in co-stimulating and co-inhibitory responses, Immunity, 44, 10051019.
Ware, C. F. (2005) Network communications: lymphotoxins, LIGHT, and TNF, Annu. Rev. Immunol., 23, 787–819.
Croft, M., Benedict, C. A., and Ware, C. F. (2013) Clinical targeting of the TNF and TNFR superfamilies, Nat. Rev. Drug Discov., 12, 147–168.
Browning, J. L., and French, L. E. (2002) Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos, J. Immunol., 168, 5079–5087.
Fu, Y. X., and Chaplin, D. D. (1999) Development and maturation of secondary lymphoid tissues, Annu. Rev. Immunol., 17, 399–433.
Sudhamsu, J., Yin, J., Chiang, E. Y., Starovasnik, M. A., Grogan, J. L., and Hymowitz, S. G. (2013) Dimerization of LTbetaR by LTalpha1beta2 is necessary and sufficient for signal transduction, Proc. Natl. Acad. Sci. USA, 110, 1989619901.
Faustman, D., and Davis, M. (2010) TNF receptor 2 pathway: drug target for autoimmune diseases, Nat. Rev. Drug Discov., 9, 482–493.
Steinberg, M. W., Cheung, T. C., and Ware, C. F. (2011) The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation, Immunol. Rev., 244, 169–187.
Randall, T. D., Carragher, D. M., and Rangel-Moreno, J. (2008) Development of secondary lymphoid organs, Annu. Rev. Immunol., 26, 627–650.
McCarthy, D. D., Summers-Deluca, L., Vu, F., Chiu, S., Gao, Y., and Gommerman, J. L. (2006) The lymphotoxin pathway: beyond lymph node development, Immunol. Res., 35, 41–54.
Lo, J. C., Wang, Y., Tumanov, A. V., Bamji, M., Yao, Z., Reardon, C. A., Getz, G. S., and Fu, Y. X. (2007) Lymphotoxin beta receptor-dependent control of lipid homeostasis, Science, 316, 285–288.
Tumanov, A. V., Christiansen, P. A., and Fu, Y.-X. (2007) The role of lymphotoxin receptor signaling in diseases, Curr. Mol. Med., 7, 567–578.
Haybaeck, J., Zeller, N., Wolf, M. J., Weber, A., Wagner, U., Kurrer, M. O., Bremer, J., Iezzi, G., Graf, R., Clavien, P. A., Thimme, R., Blum, H., Nedospasov, S. A., Zatloukal, K., Ramzan, M., Ciesek, S., Pietschmann, T., Marche, P. N., Karin, M., Kopf, M., Browning, J. L., Aguzzi, A., and Heikenwalder, M. (2009) A lymphotoxindriven pathway to hepatocellular carcinoma, Cancer Cell, 16, 295–308.
Upadhyay, V., and Fu, Y. X. (2013) Lymphotoxin signalling in immune homeostasis and the control of microorganisms, Nat. Rev. Immunol., 13, 270–279.
Kruglov, A. A., Grivennikov, S. I., Kuprash, D. V., Winsauer, C., Prepens, S., Seleznik, G. M., Eberl, G., Littman, D. R., Heikenwalder, M., Tumanov, A. V., and Nedospasov, S. A. (2013) Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis, Science, 342, 1243–1246.
Macho-Fernandez, E., Koroleva, E. P., Spencer, C. M., Tighe, M., Torrado, E., Cooper, A. M., Fu, Y. X., and Tumanov, A. V. (2015) Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells, Mucosal Immunol., 8, 403–413.
Tumanov, A. V., Grivennikov, S. I., Kruglov, A. A., Shebzukhov, Y. V., Koroleva, E. P., Piao, Y., Cui, C. Y., Kuprash, D. V., and Nedospasov, S. A. (2010) Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs, Blood, 116, 3456–3464.
Nielsen, O. H., and Ainsworth, M. A. (2013) Tumor necrosis factor inhibitors for inflammatory bowel disease, New Engl. J. Med., 369, 754–762.
Winsauer, C., Kruglov, A. A., Chashchina, A. A., Drutskaya, M. S., and Nedospasov, S. A. (2014) Cellular sources of pathogenic and protective TNF and experimental strategies based on utilization of TNF humanized mice, Cytokine Growth Factor Rev., 25, 115–123.
Dothel, G., Vasina, V., Barbara, G., and De Ponti, F. (2013) Animal models of chemically induced intestinal inflammation: predictivity and ethical issues, Pharmacol. Ther., 139, 71–86.
DeVoss, J., and Diehl, L. (2014) Murine models of inflammatory bowel disease (IBD): challenges of modeling human disease, Toxicol. Pathol., 42, 99–110.
Jiminez, J. A., Uwiera, T. C., Douglas Inglis, G., and Uwiera, R. R. (2015) Animal models to study acute and chronic intestinal inflammation in mammals, Gut Pathog., 7, 29.
Mizoguchi, A., Takeuchi, T., Himuro, H., Okada, T., and Mizoguchi, E. (2016) Genetically engineered mouse models for studying inflammatory bowel disease, J. Pathol., 238, 205–219.
Danese, S., Fiocchi, C., and Panes, J. (2016) Drug development in IBD: from novel target identification to early clinical trials, Gut, 65, 1233–1239.
Jurjus, A. R., Khoury, N. N., and Reimund, J.-M. (2004) Animal models of inflammatory bowel disease, J. Pharmacol. Toxicol. Methods, 50, 81–92.
Vorobyov, G. I., and Khalif, I. L. (eds.) (2008) Nonspecific Inflammatory Bowel Disease [in Russian], Miklosh, Moscow.
Baumgart, D. C., and Sandborn, W. J. (2012) Crohn’s disease, Lancet, 380, 1590–1605.
Danese, S., and Fiocchi, C. (2011) Ulcerative colitis, New Engl. J. Med., 365, 1713–1725.
Zimmerman, Ya. S., Zimmerman, I. Ya., and Tretyakova, Yu. I. (2013) Ulcerative colitis and Crohn’s disease: modern concept. Part 1. Definition, terminology, occurrence, etiology and pathogensis, diagnostics, complications, classification, Klin. Med., 11, 27–33.
Zimmerman, Ya. S., Zimmerman, I. Ya., and Tretyakova, Yu. I. (2013) Ulcerative colitis and Crohn’s disease: modern concept. Part 2. Diagnostics and differentiation therapy, Klin. Med., 12, 9–16.
Kaser, A., Zeissig, S., and Blumberg, R. S. (2010) Inflammatory bowel disease, Annu. Rev. Immunol., 28, 573–621.
Kaplan, G. G. (2015) The global burden of IBD: from 2015 to 2025, Nat. Rev. Gastroenterol. Hepatol., 12, 720–727.
Wallace, K. L., Zheng, L. B., Kanazawa, Y., and Shih, D. Q. (2014) Immunopathology of inflammatory bowel disease, World J. Gastroenterol., 20, 6–21.
Neurath, M. F. (2014) Cytokines in inflammatory bowel disease, Nat. Rev. Immunol., 14, 329–342.
Dalal, S. R., and Chang, E. B. (2014) The microbial basis of inflammatory bowel diseases, The J. Clin. Invest., 124, 4190–4196.
Khor, B., Gardet, A., and Xavier, R. J. (2011) Genetics and pathogenesis of inflammatory bowel disease, Nature, 474, 307–317.
Sheehan, D., Moran, C., and Shanahan, F. (2015) The microbiota in inflammatory bowel disease, J. Gastroenterol., 50, 495–507.
Wirtz, S., Neufert, C., Weigmann, B., and Neurath, M. F. (2007) Chemically induced mouse models of intestinal inflammation, Nat. Protoc., 2, 541–546.
Low, D., Nguyen, D. D., and Mizoguchi, E. (2013) Animal models of ulcerative colitis and their application in drug research, Drug Des. Devel. Ther., 7, 1341–1357.
Okayasu, I., Hatakeyama, S., Yamada, M., Ohkusa, T., Inagaki, Y., and Nakaya, R. (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice, Gastroenterology, 98, 694–702.
Pers, M., and Cerar, A. (2012) Dextran sodium sulphate colitis mouse model: traps and tricks, J. Biomed. Biotechnol., 2012, 718617.
Chassaing, B., Aitken, J. D., Malleshappa, M., and VijayKumar, M. (2014) Dextran sulfate sodium (DSS)-induced colitis in mice, Curr. Protoc. Immunol., 104, Unit 15.25.
Te Velde, A. A., Verstege, M. I., and Hommes, D. W. (2006) Critical appraisal of the current practice in murine TNBSinduced colitis, Inflamm. Bowel Dis., 12, 995–999.
Motavallian-Naeini, A., Andalib, S., Rabbani, M., Mahzouni, P., Afsharipour, M., and Minaiyan, M. (2012) Validation and optimization of experimental colitis induction in rats using 2,4,6-trinitrobenzene sulfonic acid, Res. Pharm. Sci., 7, 159–169.
Ko, J.-K.-S., Lam, F.-Y.-L., and Cheung, A.-P.-L. (2005) Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis, World J. Gastroenterol., 11, 5787–5794.
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S., and Strober, W. (2002) Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13producing NK-T cells, Immunity, 17, 629–638.
Collins, J. W., Keeney, K. M., Crepin, V. F., Rathinam, V. A., Fitzgerald, K. A., Finlay, B. B., and Frankel, G. (2014) Citrobacter rodentium: infection, inflammation and the microbiota, Nat. Rev. Microbiol., 12, 612–623.
Koroleva, E. P., Halperin, S., Gubernatorova, E. O., Macho-Fernandez, E., Spencer, C. M., and Tumanov, A. V. (2015) Citrobacter rodentium-induced colitis: a robust model to study mucosal immune responses in the gut, J. Immunol. Methods, 421, 61–72.
Rivera-Nieves, J., Bamias, G., Vidrich, A., Marini, M., Pizarro, T. T., McDuffie, M. J., Moskaluk, C. A., Cohn, S. M., and Cominelli, F. (2003) Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis, Gastroenterology, 124, 972–982.
Matsumoto, S., Okabe, Y., Setoyama, H., Takayama, K., Ohtsuka, J., Funahashi, H., Imaoka, A., Okada, Y., and Umesaki, Y. (1998) Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain, Gut, 43, 71–78.
Pizarro, T. T., Arseneau, K. O., Bamias, G., and Cominelli, F. (2003) Mouse models for the study of Crohn’s disease, Trends Mol. Med., 9, 218–222.
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K., and Muller, W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis, Cell, 75, 263–274.
Keubler, L. M., Buettner, M., Hager, C., and Bleich, A. (2015) A multihit model: colitis lessons from the interleukin-10-deficient mouse, Inflamm. Bowel Dis., 21, 19671975.
Kontoyiannis, D., Boulougouris, G., Manoloukos, M., Armaka, M., Apostolaki, M., Pizarro, T., Kotlyarov, A., Forster, I., Flavell, R., Gaestel, M., Tsichlis, P., Cominelli, F., and Kollias, G. (2002) Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease, J. Exp. Med., 196, 1563–1574.
Leach, M. W., Bean, A. G., Mauze, S., Coffman, R. L., and Powrie, F. (1996) Inflammatory bowel disease in C.B17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T-cells, Am. J. Pathol., 148, 1503–1515.
Ostanin, D. V., Bao, J., Koboziev, I., Gray, L., RobinsonJackson, S. A., Kosloski-Davidson, M., Price, V. H., and Grisham, M. B. (2009) T-cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade, Am. J. Physiol. Gastrointest. Liver Physiol., 296, G135-146.
Sadlack, B., Merz, H., Schorle, H., Schimpl, A., Feller, A. C., and Horak, I. (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene, Cell, 75, 253–261.
Baumgart, D. C., Olivier, W. A., Reya, T., Peritt, D., Rombeau, J. L., and Carding, S. R. (1998) Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice, Cell Immunol., 187, 52–66.
Lee, E. G., Boone, D. L., Chai, S., Libby, S. L., Chien, M., Lodolce, J. P., and Ma, A. (2000) Failure to regulate TNFinduced NF-kappaB and cell death responses in A20-deficient mice, Science, 289, 2350–2354.
Hammer, G. E., Turer, E. E., Taylor, K. E., Fang, C. J., Advincula, R., Oshima, S., Barrera, J., Huang, E. J., Hou, B., Malynn, B. A., Reizis, B., DeFranco, A., Criswell, L. A., Nakamura, M. C., and Ma, A. (2011) Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis, Nat. Immunol., 12, 1184–1193.
Mombaerts, P., Mizoguchi, E., Grusby, M. J., Glimcher, L. H., Bhan, A. K., and Tonegawa, S. (1993) Spontaneous development of inflammatory bowel disease in T-cell receptor mutant mice, Cell, 75, 274–282.
Nagatani, K., Wang, S., Llado, V., Lau, C. W., Li, Z., Mizoguchi, A., Nagler, C. R., Shibata, Y., Reinecker, H.C., Mora, J. R., and Mizoguchi, E. (2012) Chitin microparticles for the control of intestinal inflammation, Inflamm. Bowel Dis., 18, 1698–1710.
Nenci, A., Becker, C., Wullaert, A., Gareus, R., Van Loo, G., Danese, S., Huth, M., Nikolaev, A., Neufert, C., Madison, B., Gumucio, D., Neurath, M. F., and Pasparakis, M. (2007) Epithelial NEMO links innate immunity to chronic intestinal inflammation, Nature, 446, 557–561.
Watanabe, M., Ueno, Y., Yajima, T., Okamoto, S., Hayashi, T., Yamazaki, M., Iwao, Y., Ishii, H., Habu, S., Uehira, M., Nishimoto, H., Ishikawa, H., Hata, J., and Hibi, T. (1998) Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa, J. Exp. Med., 187, 389402.
Rath, H. C., Wilson, K. H., and Sartor, R. B. (1999) Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli, Infect. Immun., 67, 2969–2974.
Rath, H. C. (2002) Role of commensal bacteria in chronic experimental colitis: lessons from the HLA-B27 transgenic rat, Pathobiology, 70, 131–138.
Peloquin, J. M., and Nguyen, D. D. (2013) The microbiota and inflammatory bowel disease: insights from animal models, Anaerobe, 24, 102–106.
Gkouskou, K. K., Deligianni, C., Tsatsanis, C., and Eliopoulos, A. G. (2014) The gut microbiota in mouse models of inflammatory bowel disease, Front. Cell. Infect. Microbiol., 4, 28.
Eri, R., McGuckin, M. A., and Wadley, R. (2012) T cell transfer model of colitis: a great tool to assess the contribution of T cells in chronic intestinal inflammation, Methods Mol. Biol., 844, 261–275.
Shouval, D. S., Ouahed, J., Biswas, A., Goettel, J. A., Horwitz, B. H., Klein, C., Muise, A. M., and Snapper, S. B. (2014) Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans, Adv. Immunol., 122, 177–210.
Koboziev, I., Jones-Hall, Y., Valentine, J. F., Webb, C. R., Furr, K. L., and Grisham, M. B. (2015) Use of humanized mice to study the pathogenesis of autoimmune and inflammatory diseases, Inflamm. Bowel Dis., 21, 1652–1673.
Sonnenberg, G. F., and Artis, D. (2015) Innate lymphoid cells in the initiation, regulation and resolution of inflammation, Nat. Med., 21, 698–708.
Eberl, G., Colonna, M., Di Santo, J. P., and McKenzie, A. N. (2015) Innate lymphoid cells: a new paradigm in immunology, Science, 348, aaa6566.
Spits, H., and Cupedo, T. (2012) Innate lymphoid cells: emerging insights in development, lineage relationships, and function, Annu. Rev. Immunol., 30, 647–675.
Diefenbach, A., Colonna, M., and Koyasu, S. (2014) Development, differentiation, and diversity of innate lymphoid cells, Immunity, 41, 354–365.
Vivier, E., Van De Pavert, S. A., Cooper, M. D., and Belz, G. T. (2016) The evolution of innate lymphoid cells, Nat. Immunol., 17, 790–794.
Spits, H., Artis, D., Colonna, M., Diefenbach, A., Di Santo, J. P., Eberl, G., Koyasu, S., Locksley, R. M., McKenzie, A. N., Mebius, R. E., Powrie, F., and Vivier, E. (2013) Innate lymphoid cells–a proposal for uniform nomenclature, Nat. Rev. Immunol., 13, 145–149.
McKenzie, A. N., Spits, H., and Eberl, G. (2014) Innate lymphoid cells in inflammation and immunity, Immunity, 41, 366–374.
Klose, C. S., and Artis, D. (2016) Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis, Nat. Immunol., 17, 765–774.
Wang, Y., Koroleva, E. P., Kruglov, A. A., Kuprash, D. V., Nedospasov, S. A., Fu, Y. X., and Tumanov, A. V. (2010) Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection, Immunity, 32, 403–413.
Sawa, S., Lochner, M., Satoh-Takayama, N., Dulauroy, S., Berard, M., Kleinschek, M., Cua, D., Di Santo, J. P., and Eberl, G. (2011) RORgammat(+) innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota, Nat. Immunol., 12, 320–326.
Eberl, G. (2012) Development and evolution of RORgammat+ cells in a microbe’s world, Immunol. Rev., 245, 177–188.
Sonnenberg, G. F., Monticelli, L. A., Alenghat, T., Fung, T. C., Hutnick, N. A., Kunisawa, J., Shibata, N., Grunberg, S., Sinha, R., Zahm, A. M., Tardif, M. R., Sathaliyawala, T., Kubota, M., Farber, D. L., Collman, R. G., Shaked, A., Fouser, L. A., Weiner, D. B., Tessier, P. A., Friedman, J. R., Kiyono, H., Bushman, F. D., Chang, K. M., and Artis, D. (2012) Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria, Science, 336, 1321–1325.
Hepworth, M. R., Fung, T. C., Masur, S. H., Kelsen, J. R., McConnell, F. M., Dubrot, J., Withers, D. R., Hugues, S., Farrar, M. A., Reith, W., Eberl, G., Baldassano, R. N., Laufer, T. M., Elson, C. O., and Sonnenberg, G. F. (2015) Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T-cells, Science, 348, 1031–1035.
Goc, J., Hepworth, M. R., and Sonnenberg, G. F. (2016) Group 3 innate lymphoid cells: regulating host-commensal bacteria interactions in inflammation and cancer, Int. Immunol., 28, 43–52.
Cording, S., Medvedovic, J., Aychek, T., and Eberl, G. (2016) Innate lymphoid cells in defense, immunopathology and immunotherapy, Nat. Immunol., 17, 755–757.
Zheng, Y., Valdez, P. A., Danilenko, D. M., Hu, Y., Sa, S. M., Gong, Q., Abbas, A. R., Modrusan, Z., Ghilardi, N., De Sauvage, F. J., and Ouyang, W. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens, Nat. Med., 14, 282–289.
Hernandez, P. P., Mahlakoiv, T., Yang, I., Schwierzeck, V., Nguyen, N., Guendel, F., Gronke, K., Ryffel, B., Holscher, C., Dumoutier, L., Renauld, J. C., Suerbaum, S., Staeheli, P., and Diefenbach, A. (2015) Interferonlambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection, Nat. Immunol., 16, 698–707.
Vonarbourg, C., Mortha, A., Bui, V. L., Hernandez, P. P., Kiss, E. A., Hoyler, T., Flach, M., Bengsch, B., Thimme, R., Holscher, C., Honig, M., Pannicke, U., Schwarz, K., Ware, C. F., Finke, D., and Diefenbach, A. (2010) Regulated expression of nuclear receptor RORt confers distinct functional fates to NK cell receptorexpressing RORt(+) innate lymphocytes, Immunity, 33, 736–751.
Fuchs, A., Vermi, W., Lee, J. S., Lonardi, S., Gilfillan, S., Newberry, R. D., Cella, M., and Colonna, M. (2013) Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12and IL-15-responsive IFN-gamma-producing cells, Immunity, 38, 769–781.
Bernink, J. H., Peters, C. P., Munneke, M., Te Velde, A. A., Meijer, S. L., Weijer, K., Hreggvidsdottir, H. S., Heinsbroek, S. E., Legrand, N., Buskens, C. J., Bemelman, W. A., Mjosberg, J. M., and Spits, H. (2013) Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues, Nat. Immunol., 14, 221229.
Bernink, J. H., Krabbendam, L., Germar, K., De Jong, E., Gronke, K., Kofoed-Nielsen, M., Munneke, J. M., Hazenberg, M. D., Villaudy, J., Buskens, C. J., Bemelman, W. A., Diefenbach, A., Blom, B., and Spits, H. (2015) Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria, Immunity, 43, 146–160.
Spencer, S. P., Wilhelm, C., Yang, Q., Hall, J. A., Bouladoux, N., Boyd, A., Nutman, T. B., Urban, J. F., Wang, J., Ramalingam, T. R., Bhandoola, A., Wynn, T. A., and Belkaid, Y. (2014) Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity, Science, 343, 432–437.
Zook, E. C., and Kee, B. L. (2016) Development of innate lymphoid cells, Nat. Immunol., 17, 775–782.
Koues, O. I., Collins, P. L., Cella, M., Robinette, M. L., Porter, S. I., Pyfrom, S. C., Payton, J. E., Colonna, M., and Oltz, E. M. (2016) Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells, Cell, 165, 1134–1146.
Sonnenberg, G. F. (2016) Transcriptionally defining ILC heterogeneity in humans, Nat. Immunol., 17, 351–352.
Magri, G., Miyajima, M., Bascones, S., Mortha, A., Puga, I., Cassis, L., Barra, C. M., Comerma, L., Chudnovskiy, A., Gentile, M., Llige, D., Cols, M., Serrano, S., Arostegui, J. I., Juan, M., Yague, J., Merad, M., Fagarasan, S., and Cerutti, A. (2014) Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells, Nat. Immunol., 15, 354–364.
Magri, G., and Cerutti, A. (2015) Role of group 3 innate lymphoid cells in antibody production, Curr. Opin. Immunol., 33, 36–42.
Hanash, A. M., Dudakov, J. A., Hua, G., O’Connor, M. H., Young, L. F., Singer, N. V., West, M. L., Jenq, R. R., Holland, A. M., Kappel, L. W., Ghosh, A., Tsai, J. J., Rao, U. K., Yim, N. L., Smith, O. M., Velardi, E., Hawryluk, E. B., Murphy, G. F., Liu, C., Fouser, L. A., Kolesnick, R., Blazar, B. R., and Van den Brink, M. R. (2012) Interleukin-22 protects intestinal stem cells from immunemediated tissue damage and regulates sensitivity to graft versus host disease, Immunity, 37, 339–350.
Dudakov, J. A., Hanash, A. M., and Van den Brink, M. R. (2015) Interleukin-22: immunobiology and pathology, Annu. Rev. Immunol., 33, 747–785.
Buonocore, S., Ahern, P. P., Uhlig, H. H., Ivanov, I. I., Littman, D. R., Maloy, K. J., and Powrie, F. (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology, Nature, 464, 1371–1375.
Geremia, A., Arancibia-Carcamo, C. V., Fleming, M. P., Rust, N., Singh, B., Mortensen, N. J., Travis, S. P., and Powrie, F. (2011) IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease, J. Exp. Med., 208, 1127–1133.
Geremia, A., Biancheri, P., Allan, P., Corazza, G. R., and Di Sabatino, A. (2014) Innate and adaptive immunity in inflammatory bowel disease, Autoimmun. Rev., 13, 3–10.
Withers, D. R., Hepworth, M. R., Wang, X., Mackley, E. C., Halford, E. E., Dutton, E. E., Marriott, C. L., Brucklacher-Waldert, V., Veldhoen, M., Kelsen, J., Baldassano, R. N., and Sonnenberg, G. F. (2016) Transient inhibition of ROR-gammat therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells, Nat. Med., 22, 319–323.
Goldberg, R., Prescott, N., Lord, G. M., MacDonald, T. T., and Powell, N. (2015) The unusual suspects–innate lymphoid cells as novel therapeutic targets in IBD, Nat. Rev. Gastroenterol. Hepatol., 12, 271–283.
Tumanov, A. V., Koroleva, E. P., Guo, X., Wang, Y., Kruglov, A., Nedospasov, S., and Fu, Y. X. (2011) Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge, Cell Host Microbe, 10, 44–53.
Spahn, T. W., Maaser, C., Eckmann, L., Heidemann, J., Lugering, A., Newberry, R., Domschke, W., Herbst, H., and Kucharzik, T. (2004) The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentiuminduced colitis, Gastroenterology, 127, 1463.
Cerovic, V., Bain, C. C., Mowat, A. M., and Milling, S. W. (2014) Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol., 35, 270–277.
Guilliams, M., Ginhoux, F., Jakubzick, C., Naik, S. H., Onai, N., Schraml, B. U., Segura, E., Tussiwand, R., and Yona, S. (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny, Nat. Rev. Immunol., 14, 571–578.
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., and Gordon, J. I. (2005) Host-bacterial mutualism in the human intestine, Science, 307, 1915–1920.
Gensollen, T., Iyer, S. S., Kasper, D. L., and Blumberg, R. S. (2016) How colonization by microbiota in early life shapes the immune system, Science, 352, 539–544.
Caballero, S., and Pamer, E. G. (2015) Microbiota-mediated inflammation and antimicrobial defense in the intestine, Annu. Rev. Immunol., 33, 227–256.
Hamada, H., Hiroi, T., Nishiyama, Y., Takahashi, H., Masunaga, Y., Hachimura, S., Kaminogawa, S., Takahashi-Iwanaga, H., Iwanaga, T., Kiyono, H., Yamamoto, H., and Ishikawa, H. (2002) Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine, J. Immunol., 168, 57–64.
Mantis, N. J., McGuinness, C. R., Sonuyi, O., Edwards, G., and Farrant, S. A. (2006) Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication, Infect. Immun., 74, 3455–3462.
Pabst, O. (2012) New concepts in the generation and functions of IgA, Nat. Rev. Immunol., 12, 821–832.
Bemark, M., Boysen, P., and Lycke, N. Y. (2012) through T cell-dependent and T-cell-independent pathways, Ann. N. Y. Acad. Sci., 1247, 97–116.
Cerutti, A. (2008) The regulation of IgA class switching, Nat. Rev. Immunol., 8, 421–434.
Gutzeit, C., Magri, G., and Cerutti, A. (2014) Intestinal IgA production and its role in host-microbe interaction, Immunol. Rev., 260, 76–85.
Fagarasan, S., Kawamoto, S., Kanagawa, O., and Suzuki, K. (2010) Adaptive immune regulation in the gut: T-celldependent and T-cell-independent IgA synthesis, Annu. Rev. Immunol., 28, 243–273.
Masahata, K., Umemoto, E., Kayama, H., Kotani, M., Nakamura, S., Kurakawa, T., Kikuta, J., Gotoh, K., Motooka, D., Sato, S., Higuchi, T., Baba, Y., Kurosaki, T., Kinoshita, M., Shimada, Y., Kimura, T., Okumura, R., Takeda, A., Tajima, M., Yoshie, O., Fukuzawa, M., Kiyono, H., Fagarasan, S., Iida, T., Ishii, M., and Takeda, K. (2014) Generation of colonic IgA-secreting cells in the caecal patch, Nat. Commun., 5, 3704.
Macpherson, A. J., Gatto, D., Sainsbury, E., Harriman, G. R., Hengartner, H., and Zinkernagel, R. M. (2000) A primitive T-cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria, Science, 288, 2222–2226.
Mora, J. R., Iwata, M., Eksteen, B., Song, S. Y., Junt, T., Senman, B., Otipoby, K. L., Yokota, A., Takeuchi, H., Ricciardi-Castagnoli, P., Rajewsky, K., Adams, D. H., and Von Andrian, U. H. (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells, Science, 314, 1157–1160.
Lorenz, R. G., Chaplin, D. D., McDonald, K. G., McDonough, J. S., and Newberry, R. D. (2003) Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function, J. Immunol., 170, 5475–5482.
Van De Pavert, S. A., and Mebius, R. E. (2010) New insights into the development of lymphoid tissues, Nat. Rev. Immunol., 10, 664–674.
Bar-Ephraim, Y. E., and Mebius, R. E. (2016) Innate lymphoid cells in secondary lymphoid organs, Immunol. Rev., 271, 185–199.
Kuprash, D. V., Tumanov, A. V., Liepinsh, D. J., Koroleva, E. P., Drutskaya, M. S., Kruglov, A. A., Shakhov, A. N., Southon, E., Murphy, W. J., Tessarollo, L., Grivennikov, S. I., and Nedospasov, S. A. (2005) Novel tumor necrosis factor-knockout mice that lack Peyer’s patches, Eur. J. Immunol., 35, 1592–1600.
Kang, H. S., Chin, R. K., Wang, Y., Yu, P., Wang, J., Newell, K. A., and Fu, Y. X. (2002) Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production, Nat. Immunol., 3, 576–582.
Danese, S., and Peyrin-Biroulet, L. (2014) IBD in 2013: enriching the therapeutic armamentarium for IBD, Nat. Rev. Gastroenterol. Hepatol., 11, 84–86.
Rogler, G. (2015) Where are we heading to in pharmacological IBD therapy? Pharmacol. Res., 100, 220–227.
Scharl, M., and Rogler, G. (2012) Inflammatory bowel disease pathogenesis: what is new? Curr. Opin. Gastroenterol., 28, 301–309.
Bosani, M., Ardizzone, S., and Porro, G. B. (2009) Biologic targeting in the treatment of inflammatory bowel diseases, Biologics, 3, 77–97.
Olesen, C. M., Coskun, M., Peyrin-Biroulet, L., and Nielsen, O. H. (2016) Mechanisms behind efficacy of tumor necrosis factor inhibitors in inflammatory bowel diseases, Pharmacol. Ther., 159, 110–119.
Murch, S. H., Lamkin, V. A., Savage, M. O., WalkerSmith, J. A., and MacDonald, T. T. (1991) Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease, Gut, 32, 913–917.
Breese, E. J., Michie, C. A., Nicholls, S. W., Murch, S. H., Williams, C. B., Domizio, P., Walker-Smith, J. A., and MacDonald, T. T. (1994) Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease, Gastroenterology, 106, 1455–1466.
Nilsen, E. M., Johansen, F. E., Kvale, D., Krajci, P., and Brandtzaeg, P. (1999) Different regulatory pathways employed in cytokine-enhanced expression of secretory component and epithelial HLA class I genes, Eur. J. Immunol., 29, 168–179.
D’Haens, G. (2010) Anti-TNF treatment in Crohn’s disease: toward tailored therapy? Am. J. Gastroenterol., 105, 1140–1141.
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G., and Tak, P. P. (2008) Tumor necrosis factor antagonist mechanisms of action: a comprehensive review, Pharmacol. Ther., 117, 244–279.
Ueda, N., Tsukamoto, H., Mitoma, H., Ayano, M., Tanaka, A., Ohta, S., Inoue, Y., Arinobu, Y., Niiro, H., Akashi, K., and Horiuchi, T. (2013) The cytotoxic effects of certolizumab pegol and golimumab mediated by transmembrane tumor necrosis factor alpha, Inflamm. Bowel Dis., 19, 1224–1231.
Slevin, S. M., and Egan, L. J. (2015) New insights into the mechanisms of action of anti-tumor necrosis factor-alpha monoclonal antibodies in inflammatory bowel disease, Inflamm. Bowel Dis., 21, 2909–2920.
Shchigoleva, N. E., Matina, I. A., Ponomariova, A. P., Karpina, L. M., and Bologov, A. A. (2010) Use of infliximab in children with inflammatory bowel diseases, Pediatr. Pharmacol., 7, 55–61.
Benucci, M., Saviola, G., Manfredi, M., Sarzi-Puttini, P., and Atzeni, F. (2012) Tumor necrosis factors blocking agents: analogies and differences, Acta Biomed., 83, 72–80.
Thorlund, K., Druyts, E., Toor, K., and Mills, E. J. (2015) Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs, Expert. Rev. Gastroenterol. Hepatol., 9, 693–700.
Peake, S. T., Bernardo, D., Mann, E. R., Al-Hassi, H. O., Knight, S. C., and Hart, A. L. (2013) Mechanisms of action of anti-tumor necrosis factor alpha agents in Crohn’s disease, Inflamm. Bowel Dis., 19, 1546–1555.
Steenholdt, C., Brynskov, J., Thomsen, O. O., Munck, L. K., Fallingborg, J., Christensen, L. A., Pedersen, G., Kjeldsen, J., Jacobsen, B. A., Oxholm, A. S., Kjellberg, J., Bendtzen, K., and Ainsworth, M. A. (2014) Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial, Gut, 63, 919–927.
Kaymakcalan, Z., Sakorafas, P., Bose, S., Scesney, S., Xiong, L., Hanzatian, D. K., Salfeld, J., and Sasso, E. H. (2009) Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor, Clin. Immunol., 131, 308–316.
Shealy, D. J., Cai, A., Staquet, K., Baker, A., Lacy, E. R., Johns, L., Vafa, O., Gunn, G., 3rd, Tam, S., Sague, S., Wang, D., Brigham-Burke, M., Dalmonte, P., Emmell, E., Pikounis, B., Bugelski, P. J., Zhou, H., Scallon, B. J., and Giles-Komar, J. (2010) Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor alpha, MAbs, 2, 428–439.
Scallon, B., Cai, A., Solowski, N., Rosenberg, A., Song, X. Y., Shealy, D., and Wagner, C. (2002) Binding and functional comparisons of two types of tumor necrosis factor antagonists, J. Pharmacol. Exp. Ther., 301, 418–426.
Nesbitt, A., Fossati, G., Bergin, M., Stephens, P., Stephens, S., Foulkes, R., Brown, D., Robinson, M., and Bourne, T. (2007) Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other antitumor necrosis factor alpha agents, Inflamm. Bowel Dis., 13, 1323–1332.
Vos, A. C., Wildenberg, M. E., Arijs, I., Duijvestein, M., Verhaar, A. P., De Hertogh, G., Vermeire, S., Rutgeerts, P., Van den Brink, G. R., and Hommes, D. W. (2012) Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro, Inflamm. Bowel Dis., 18, 401–408.
Perrier, C., De Hertogh, G., Cremer, J., Vermeire, S., Rutgeerts, P., Van Assche, G., Szymkowski, D. E., and Ceuppens, J. L. (2013) Neutralization of membrane TNF, but not soluble TNF, is crucial for the treatment of experimental colitis, Inflamm. Bowel Dis., 19, 246–253.
Oikonomopoulos, A., Van Deen, W. K., and Hommes, D. W. (2013) Anti-TNF antibodies in inflammatory bowel disease: do we finally know how it works? Curr. Drug Targets, 14, 1421–1432.
Sandborn, W. J., Hanauer, S. B., Katz, S., Safdi, M., Wolf, D. G., Baerg, R. D., Tremaine, W. J., Johnson, T., Diehl, N. N., and Zinsmeister, A. R. (2001) Etanercept for active Crohn’s disease: a randomized, double-blind, placebocontrolled trial, Gastroenterology, 121, 1088–1094.
Van den Brande, J. M., Braat, H., Van den Brink, G. R., Versteeg, H. H., Bauer, C. A., Hoedemaeker, I., Van Montfrans, C., Hommes, D. W., Peppelenbosch, M. P., and Van Deventer, S. J. (2003) Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease, Gastroenterology, 124, 1774–1785.
Mocci, G., Marzo, M., Papa, A., Armuzzi, A., and Guidi, L. (2013) Dermatological adverse reactions during antiTNF treatments: focus on inflammatory bowel disease, J. Crohn’s Colitis, 7, 769–779.
Targownik, L. E., and Bernstein, C. N. (2013) Infectious and malignant complications of TNF inhibitor therapy in IBD, Am. J. Gastroenterol., 108, 1835–1842, quiz 1843.
Cleynen, I., and Vermeire, S. (2012) Paradoxical inflammation induced by anti-TNF agents in patients with IBD, Nat. Rev. Gastroenterol. Hepatol., 9, 496–503.
Wiens, A., Venson, R., Correr, C. J., Otuki, M. F., and Pontarolo, R. (2010) Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis, Pharmacotherapy, 30, 339–353.
Heldmann, F., Brandt, J., Van der Horst-Bruinsma, I. E., Landewe, R., Sieper, J., Burmester, G. R., Van den Bosch, F., De Vlam, K., Geusens, P., Gaston, H., Schewe, S., Appelboom, T., Emery, P., Dougados, M., Leirisalo-Repo, M., Breban, M., Listing, J., and Braun, J. (2011) The European ankylosing spondylitis infliximab cohort (EASIC): a European multicentre study of long term outcomes in patients with ankylosing spondylitis treated with infliximab, Clin. Exp. Rheumatol., 29, 672–680.
Tzu, J., and Kerdel, F. (2008) From conventional to cutting edge: the new era of biologics in treatment of psoriasis, Dermatol. Ther., 21, 131–141.
Angelucci, E., Cocco, A., Viscido, A., Vernia, P., and Caprilli, R. (2007) Another paradox in Crohn’s disease: new onset of psoriasis in a patient receiving tumor necrosis factor-alpha antagonist, Inflamm. Bowel Dis., 13, 10591061.
Cullen, G., Kroshinsky, D., Cheifetz, A. S., and Korzenik, J. R. (2011) Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: a new series and a review of 120 cases from the literature, Aliment. Pharmacol. Ther., 34, 1318–1327.
Palucka, A. K., Blanck, J. P., Bennett, L., Pascual, V., and Banchereau, J. (2005) Cross-regulation of TNF and IFNalpha in autoimmune diseases, Proc. Natl. Acad. Sci. USA, 102, 3372–3377.
Williams, V. L., and Cohen, P. R. (2011) TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists, Int. J. Dermatol., 50, 619–625.
Bout-Tabaku, S., Rivas-Chacon, R., and Restrepo, R. (2007) Systemic lupus erythematosus in a patient treated with etanercept for polyarticular juvenile rheumatoid arthritis, J. Rheumatol., 34, 2503–2504.
Ferraccioli, G., Mecchia, F., Di Poi, E., and Fabris, M. (2002) Anticardiolipin antibodies in rheumatoid patients treated with etanercept or conventional combination therapy: direct and indirect evidence for a possible association with infections, Ann. Rheum. Dis., 61, 358–361.
Via, C. S., Shustov, A., Rus, V., Lang, T., Nguyen, P., and Finkelman, F. D. (2001) In vivo neutralization of TNFalpha promotes humoral autoimmunity by preventing the induction of CTL, J. Immunol., 167, 6821–6826.
Fidder, H., Schnitzler, F., Ferrante, M., Noman, M., Katsanos, K., Segaert, S., Henckaerts, L., Van Assche, G., Vermeire, S., and Rutgeerts, P. (2009) Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study, Gut, 58, 501–508.
Hochman, D., and Wolff, B. (2006) Risk of serious infections and malignancies with anti-TNF antibody therapy in rheumatoid arthritis, JAMA, 296, 2203; author reply 2203-2204.
Stern, R. S., Liebman, E. J., and Vakeva, L. (1998) Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. PUVA Follow-up Study, J. Natl. Cancer Inst., 90, 1278–1284.
Peyrin-Biroulet, L., Khosrotehrani, K., Carrat, F., Bouvier, A. M., Chevaux, J. B., Simon, T., Carbonnel, F., Colombel, J. F., Dupas, J. L., Godeberge, P., Hugot, J. P., Lemann, M., Nahon, S., Sabate, J. M., Tucat, G., and Beaugerie, L. (2011) Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease, Gastroenterology, 141, 1621–1628.
Steenholdt, C., Svenson, M., Bendtzen, K., Thomsen, O. O., Brynskov, J., and Ainsworth, M. A. (2011) Severe infusion reactions to infliximab: aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease, Aliment. Pharmacol. Ther., 34, 51–58.
Slifman, N. R., Gershon, S. K., Lee, J. H., Edwards, E. T., and Braun, M. M. (2003) Listeria monocytogenes infection as a complication of treatment with tumor necrosis factor alpha-neutralizing agents, Arthritis. Rheum., 48, 319–324.
Peyrin-Biroulet, L., Deltenre, P., De Suray, N., Branche, J., Sandborn, W. J., and Colombel, J. F. (2008) Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials, Clin. Gastroenterol. Hepatol., 6, 644–653.
Shebzukhov, Y. V., Kuchmiy, A. A., Kruglov, A. A., Zipp, F., Siffrin, V., and Nedospasov, S. A. (2014) Experimental applications of TNF-reporter mice with far-red fluorescent label, Methods Mol. Biol., 1155, 151–162.
Lowenberg, M., and D’Haens, G. (2015) Next-generation therapeutics for IBD, Curr. Gastroenterol. Rep., 17, 21.
Gomez-Gomez, G. J., Masedo, A., Yela, C., MartinezMontiel Mdel, P., and Casis, B. (2015) Current stage in inflammatory bowel disease: what is next? World J. Gastroenterol., 21, 11282–11303.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E. О. Gubernatorova, A. V. Tumanov, 2016, published in Biokhimiya, 2016, Vol. 81, No. 11, pp. 1559–1577.
Rights and permissions
About this article
Cite this article
Gubernatorova, E.O., Tumanov, A.V. Tumor necrosis factor and lymphotoxin in regulation of intestinal inflammation. Biochemistry Moscow 81, 1309–1325 (2016). https://doi.org/10.1134/S0006297916110092
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916110092