Abstract
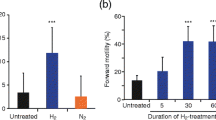
The effect of low concentrations of hydrogen peroxide (10–100 µM) on sperm motility and on the activity of the sperm enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDS) was investigated. Incubation of semen samples with 10 and 100 µM hydrogen peroxide increased the content of spermatozoa with progressive motility by 20 and 18%, respectively, and enhanced the activity of GAPDS in the sperm cells by 27 and 20% compared to a semen sample incubated without additions. It was also found that incubation with 10 µM hydrogen peroxide increased the content of reduced glutathione (GSH) in sperm cells by 50% on average compared to that in the control samples. It is supposed that low concentrations of hydrogen peroxide activate the pentose phosphate pathway, resulting in NADPH synthesis and the reduction of the oxidized glutathione by glutathione reductase yielding GSH. The formed GSH reduces the oxidized cysteine residues of the GAPDS active site, increasing the activity of the enzyme, which in turn enhances the content of sperm cells with progressive motility. Thus, the increase in motile spermatozoa in the presence of low concentrations of hydrogen peroxide can serve as an indicator of normal functioning of the antioxidant defense system in sperm cells.
Similar content being viewed by others
Abbreviations
- ATM:
-
serine/threonine protein kinase
- dN-GAPDS:
-
recombinant GAPDS without N-terminal domain
- GAPDS:
-
sperm-specific glyceraldehyde-3-phosphate dehydrogenase
- G6PD:
-
glucose-6-phosphate dehydrogenase
- PPP:
-
pentose phosphate pathway
- ROS:
-
reactive oxygen species
References
Mazzilli, F., Rossi, T., Marchesini, M., Ronconi, C., and Dondero, F. (1994) Superoxide anion in human semen related to seminal parameters and clinical aspects, Fertil. Steril., 62, 862–868.
Sharma, R. K., and Agarwal, A. (1996) Role of reactive oxygen species in male infertility, Urology, 48, 835–850.
Aitken, R. J., and Sawyer, D. (2003) The human spermatozoon — not waving but drowning, Adv. Exp. Med. Biol., 518, 85–98.
Agarwal, A., Saleh, R. A., and Bedaiwy, M. A. (2003) Role of reactive oxygen species in the pathophysiology of human reproduction, Fertil. Steril., 79, 829–843.
Elkina, Yu. L., Atroshchenko, M. M., Bragina, E. E., Muronetz, V. I., and Schmalhausen, E. V. (2011) Oxidation of glyceraldehyde-3-phosphate dehydrogenase decreases sperm motility, Biochemistry (Moscow), 76, 268–272.
Westhoff, W., and Kamp, G. (1997) Glyceraldehyde 3phosphate dehydrogenase is bound to the fibrous sheath of mammalian spermatozoa, J. Cell Sci., 110, 1821–1829.
Welch, J. E., Brown, P. L., O’ Brien, D. A., Magyar, P. L., Bunch, D. O., Mori, C., and Eddy, E. M. (2000) Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells, J. Androl., 21, 328–338.
Tanii, I., Yagura, T., Inagaki, N., and Yoshinaga, K. (2007) Preferential localization of rat GAPDS on the ribs of fibrous sheath of sperm flagellum and its expression during flagellar formation, Acta Histochem. Cytochem., 40, 19–26.
Miki, K., Ou, W., Goulding, E., Willis, W. D., Bunch, D. O., Stadler, L. F., Perreault, S. D., Eddy, E. M., and O’Brien, D. A. (2004) Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility, Proc. Natl. Acad. Sci. USA, 101, 16501–16506.
Little, C., and O’Brien, P. J. (1969) Mechanism of peroxide-inactivation of the sulfhydryl enzyme glyceraldehyde3-phosphate dehydrogenase, Eur. J. Biochem., 10, 533–538.
Schmalhausen, E. V., Nagradova, N. K., Boschi-Muller, S., Branlant, G., and Muronetz, V. I. (1999) Mildly oxidized GAPDH: the coupling of the dehydrogenase and acyl phosphatase activities, FEBS Lett., 452, 219–222.
Elkina, Yu. L., Kuravsky, M. L., El’darov, M. A., Stogov, S. V., Muronetz, V. I., and Schmalhausen, E. V. (2010) Recombinant human sperm-specific glyceraldehyde-3phosphate dehydrogenase: structural basis for enhanced stability, Biochim. Biophys. Acta, 1804, 2207–2212.
Aitken, R. J., Paterson, M., Fisher, H., Buckingham, D. W., and van Duin, M. (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function, J. Cell Sci., 108, 2017–2025.
Rivlin, J., Mendel, J., Rubinstein, S., Etkovitz, N., and Breitbart, H. (2004) Role of hydrogen peroxide in sperm capacitation and acrosome reaction, Biol. Reprod., 70, 518–522.
Shahar, S., Wiser, A., Ickowicz, D., Lubart, R., Shulman, A., and Breitbart, H. (2011) Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility, Hum. Reprod., 26, 2274–2282.
Dan’shina, P. V., Schmalhausen, E. V., Avetisyan, A. V., and Muronetz, V. I. (2001) Mildly oxidized glyceraldehyde-3-phosphate dehydrogenase as a possible regulator of glycolysis, IUBMB Life, 51, 309–314.
Bradford, M. M. (1976) A rapid and sensitive method of quantitation of microgram quantities of protein utilizing the principle of protein-due binding, Anal. Biochem., 72, 248–254.
Shchutskaya, Y. Y., Elkina, Y. L., Kuravsky, M. L., Bragina, E. E., and Schmalhausen, E. V. (2008) Investigation of glyceraldehyde-3-phosphate dehydrogenase from human sperms, Biochemistry (Moscow), 73, 185–191.
Williams, A. C., and Ford, W. C. L. (2004) Functional significance of the pentose phosphate pathway and glutathione reductase in the antioxidant defenses of human sperm, Biol. Reprod., 71, 1309–1316.
Stanton, R. S. (2012) Glucose-6-phosphate dehydrogenase, NADPH, and cell survival, IUBMB Life, 64, 362–369.
Rotman, G., and Shiloh, Y. (1997) The ATM gene and protein: possible roles in genome surveillance, checkpoint controls and cellular defense against oxidative stress, Cancer Surv., 29, 285–304.
Rotman, G., and Shiloh, Y. (1997) Ataxia-telangiectasia: is ATM a sensor of oxidative damage and stress? BioEssays, 19, 911–917.
Shiloh, Y. (2006) The ATM-mediated DNA-damage response: taking shape, Trends Biochem. Sci., 31, 402–410.
Reichenbach, J., Schubert, R., Schindler, D., Muller, K., Bohles, H., and Zielen, S. (2002) Elevated oxidative stress in patients with ataxia telangiectasia, Antioxid. Redox Signal., 4, 465–469.
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D., and Paull, T. T. (2010) ATM activation by oxidative stress, Science, 330, 517–521.
Preville, X., Salvemini, F., Giraud, S., Chaufour, S., Paul, C., Stepien, G., Ursini, M. V., and Arrigo, A. P. (1999) Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery, Exp. Cell Res., 247, 61–78.
Cosentino, C., Grieco, D., and Costanzo, V. (2011) ATM activates the pentose phosphate pathway promoting antioxidant defense and DNA repair, EMBO J., 30, 546–555.
Stanton, R. C., Seifter, J. L., Boxer, D. C., Zimmerman, E., and Cantley, L. C. (1991) Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. Correlation with increased enzymatic activity, J. Biol. Chem., 266, 12442–12448.
Swezey, R. R., and Epel, D. (1986) Regulation of glucose6-phosphate dehydrogenase activity in sea urchin eggs by reversible association with cell structural elements, J. Cell Biol., 103, 1509–1515.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2015, Vol. 80, No. 9, pp. 1431–1439.
Rights and permissions
About this article
Cite this article
Evdokimov, V.V., Barinova, K.V., Turovetskii, V.B. et al. Low concentrations of hydrogen peroxide activate the antioxidant defense system in human sperm cells. Biochemistry Moscow 80, 1178–1185 (2015). https://doi.org/10.1134/S0006297915090084
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915090084