Abstract
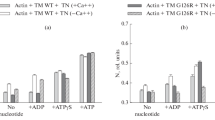
Polarized fluorimetry was used to study in ghost muscle fibers the influence of a 40-kDa protein from the thin filaments of the mussel Crenomytilus grayanus on conformational changes of F-actin modified by the fluorescent probes 1,5-IAEDANS and FITC-phalloidin during myosin subfragment (S1) binding in the absence of nucleotides and in the presence of MgADP or MgATP. The fluorescence probes were rigidly bound with actin, which made the absorption and emission dipoles of the probes sensitive to changes in the orientation and mobility of both actin monomer and its subdomain-1 in thin filaments of the muscle fiber. On modeling different intermediate states of actomyosin, the orientation and mobility of oscillators of the dyes were changed discretely, which suggests multistep changes in the actin conformation during the cycle of ATP hydrolysis. The 40-kDa protein influenced the orientation and mobility of the fluorescent probes markedly, suppressing changes in their orientation and mobility in the absence of nucleotides and in the presence of MgADP, but enhancing these changes in the presence of MgATP. The calponin-like 40-kDa protein is supposed to prevent formation of the strong binding state of actomyosin in the absence of nucleotides and in the presence of MgADP but to activate formation of this state in the presence of MgATP.
Similar content being viewed by others
Abbreviations
- A·M:
-
actomyosin
- DTT:
-
dithiothreitol
- FITC:
-
fluorescein isothiocyanate
- 1,5-IAEDANS:
-
N-iodoacetyl-N′-(5-sulfo-1-naphtyl)ethylenediamine
References
Adelstein, R. S., and Eisenberg, E. (1980) Annu. Rev. Biochem., 49, 921–956.
Somlyo, A. P., and Somlyo, A. V. (1994) Nature, 372, 231–236.
Chalovich, J. M. (1992) Pharmacol. Ther., 55, 95–148.
Hodgkinson, J. L. (2000) J. Muscle Res. Cell Motil., 21, 115–130.
Morgan, K. G., and Gangopadhyay, S. S. (2001) J. Appl. Physiol., 91, 953–962.
Lehman, W. (1991) J. Muscle Res. Cell Motil., 12, 221–224.
Winder, S. J., Allen, B. G., Clement-Chomienne, O., and Walsh, M. P. (1998) Acta Physiol. Scand., 164, 415–426.
Takahashi, K., Hiwada, K., and Kokubu, T. (1987) Life Sci., 41, 291–296.
Gimona, M., Herzog, M., Vandekerckhove, J., and Small, J. V. (1990) FEBS Lett., 274, 159–162.
Saamaha, F. F., Ip, H. S., Morrisey, E. E., Seltzer, J., Tang, Z., Solway, J., and Parmacek, M. S. (1996) J. Biol. Chem., 271, 395–403.
Ferjani, I., Fattoum, A., Maciver, S. K., Manai, M., Benyamin, Y., and Roustan, C. (2006) FEBS Lett., 580, 4801–4806.
Jaworowski, A., Anderson, K. I., Arner, A., Engstrom, M., Gimona, M., Strasser, P., and Small, J. V. (1995) FEBS Lett., 365, 167–171.
Obara, K., Szymanski, P. T., Tao, T., and Paul, R. J. (1996) Am. J. Physiol., 270, 481–487.
Takahashi, K., Hiwada, K., and Kokubu, T. (1986) Biochem. Biophys. Res. Commun., 141, 20–26.
Winder, S. J., and Walsh, M. P. (1990) J. Biol. Chem., 265, 10148–10155.
Horiuchi, K. Y., and Chacko, S. (1991) Biochem. Biophys. Res. Commun., 176, 1487–1493.
Shirinsky, V. P., Biryukov, K. G., Hettasch, J. M., and Sellers, J. R. (1992) J. Biol. Chem., 267, 15886–15892.
Haeberle, J. R. (1994) J. Biol. Chem., 269, 12424–12431.
Borovikov, Y. S., Horiuchi, K. Y., Avrova, S. V., and Chacko, S. (1996) Biochemistry, 35, 13849–13857.
Makuch, R., Birukov, K., Shirinsky, V., and Dabrowska, R. (1991) Biochem. J., 280, 33–38.
Szymanski, P. T. (2004) J. Muscle Res. Cell Motil., 25, 7–19.
Marston, S. B., and Redwood, C. S. (1991) Biochem. J., 279, 1–16.
Marston, S. B., and Redwood, C. S. (1993) J. Biol. Chem., 268, 12317–12320.
Noda, S., Ito, M., Watanabe, S., Takahashi, K., and Maruyama, K. (1992) Biochem. Biophys. Res. Commun., 185, 481–487.
Dobrzhanskaya, A. V., Matusovskaya, G. G., Matusovsky, O. S., and Shelud’ko, N. S. (2010) Biofizika, 55, 785–789.
Borovikov, Y. S., Chernogryadskaya, N. A., and Rozanov, Y. M. (1974) Tsitologiya, 16, 972–982.
Yanagida, T., and Oosawa, F. (1978) J. Mol. Biol., 126, 507–524.
Prochniewicz-Nakayama, E., Yanagida, T., and Oosawa, F. (1983) J. Cell Biol., 97, 1663–1667.
Borovikov, Y. S. (1998) Tsitologiya, 40, 715–734.
Sirenko, V. V., Simonyan, A. O., Dobrzhanskaya, A. V., Shelud’ko, N. S., and Borovikov, Y. S. (2012) Biochemistry (Moscow), 77, 889–895.
Spudich, J. A., and Watt, S. (1971) J. Biol. Chem., 246, 4866–4871.
Miki, M., dos Remedios, C. G., and Barden, J. A. (1987) Eur. J. Biochem., 168, 339–345.
Hudson, E. N., and Weber, G. (1973) Biochemistry, 12, 4154–4161.
Ivanov, I. I., and Yur’ev, V. A. (1961) Biochemistry and Pathobiochemistry of Muscles [in Russian], Meditsina, Leningrad.
Okamoto, Y., and Sekine, T. (1985) J. Biochem., 98, 1143–1145.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem., 193, 265–275.
Lehrer, S. S., and Kerwar, G. (1972) Biochemistry, 11, 1211–1217.
Verpoorte, J. A., and Kay, C. M. (1966) Arch. Biochem. Biophys., 113, 53–63.
Eisenberg, E., and Moos, C. (1968) Biochemistry, 7, 1486–1489.
Szent-Gyorgyi, A. (1949) Biol. Bull., 96, 140–161.
Borovikov, Y. S., and Gusev, N. B. (1983) Eur. J. Biochem., 136, 363–369.
Borovikov, Y. S., Dobrovolsky, Z., and Dabrowska, R. (1988) Tsitologiya, 30, 1014–1017.
Borovikov, Y. S., Nowak, E., Khoroshev, M. I., and Dabrowska, R. (1993) Biochim. Biophys. Acta, 1163, 280–286.
Nowak, E., Borovikov, Y. S., and Dabrowska, R. (1989) Biochim. Biophys. Acta, 999, 289–292.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Ioffe, V. A., Borovikov, Y. S., Barsky, I. Y., and Rozanov, Y. M. (1974) Tsitologiya, 16, 112–116.
Tregear, R., and Mendelson, R. A. (1975) Biophys. J., 15, 455–467.
Kakol, I., Borovikov, Y. S., Szczesna, D., Kirillina, V. P., and Levitsky, D. I. (1987) Biochim. Biophys. Acta, 913, 1–9.
Cooke, R. (1997) Physiol. Rev., 77, 671–697.
Higuchi, S., Tsukasaki, Y., Fukuda, N., Kurihara, S., and Fujita, H. (2011) J. Biomed. Biotechnol., 2011, id 486021.
Borovikov, Y. S., Moraczewska, J., Khoroshev, M. I., and Strzelecka-Golaszewska, H. (2000) Biochim. Biophys. Acta, 1478, 138–151.
Khaimina, S. S., Vrzhosek, A., Dabrowska, R., and Borovikov, Y. S. (2005) Biochemistry (Moscow), 70, 1136–1139.
Nagashima, H., and Asakura, S. (1982) J. Mol. Biol., 155, 409–428.
Borovikov, Y. S., Dedova, I. V., dos Remedios, C. G., Vikhoreva, N. N., Vikhorev, P. G., Avrova, S. V., Hazlett, T. L., and Van Der Meer, B. W. (2004) Biophys. J., 86, 3020–3029.
Kabsch, W., Mannherz, H. G., Suck, D., Pai, E. F., and Holmes, K. C. (1990) Nature, 347, 37–44.
Holmes, K. C., Tiron, M., Lorenz, M., Popp, D., and Kabsch, W. (1992) in Muscle as a Machine: Energy Transduction in the Contractile System (Morales, M., ed.) Bethesda, pp. 53–54.
Nowak, E., Borovikov, Y. S., Khoroshev, M. I., and Dabrowska, R. (1991) FEBS Lett., 281, 51–54.
Borovikov, Y. S. (1992) in Muscle as a Machine: Energy Transduction in the Contractile System (Morales, M., ed.) Bethesda, pp. 30–32.
Chalovich, J. M., Greene, L. E., and Eisenberg, E. (1983) Proc. Natl. Acad. Sci. USA, 80, 4909–4913.
Botts, J., Thomason, J. F., and Morales, M. F. (1989) Proc. Natl. Acad. Sci. USA, 86, 2204–2208.
Johnson, W. C., Bivin, D. B., Ue, K., and Morales, M. F. (1991) Proc. Natl. Acad. Sci. USA, 88, 9748–9750.
Cooke, R. (1995) FASEB J., 9, 636–642.
Dancker, P., Low, I., Hasselbach, W., and Wieland, T. (1975) Biochim. Biophys Acta, 400, 407–414.
Vikhorev, P. G., Vikhoreva, N. N., Roslyakova, M. A., Chako, S., and Borovikov, Y. S. (2000) Tsitologiya, 42, 444–453.
Lorenz, M., Popp, D., and Holmes, K. C. (1993) J. Mol. Biol., 234, 826–836.
Borovikov, Y. S., Avrova, S. V., Vikhoreva, N. N., Vikhorev, P. G., Ermakov, V. S., Copeland, O., and Marston, S. B. (2001) Int. J. Biochem. Cell Biol., 33, 1151–1159.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V. V. Sirenko, A. H. Simonyan, A. V. Dobrzhanskaya, N. S. Shelud’ko, Y. S. Borovikov, 2013, published in Biokhimiya, 2013, Vol. 78, No. 3, pp. 364–374.
Rights and permissions
About this article
Cite this article
Sirenko, V.V., Simonyan, A.H., Dobrzhanskaya, A.V. et al. 40-kDa protein from thin filaments of the mussel Crenomytilus grayanus changes the conformation of F-actin during the ATPase cycle. Biochemistry Moscow 78, 273–281 (2013). https://doi.org/10.1134/S0006297913030097
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297913030097