Abstract
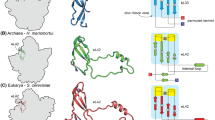
This review is devoted to substantiation of new characteristics for classification of living organisms. The novel view of a role of flexible regions in protein functioning and evolution is suggested. It is based on the newly revealed correlation between the number of loops in elongation factors and the complexity of organisms. This correlation allowed us to formulate a hypothesis of evolution of this protein family. In addition, the study of the ribosomal protein S1 family made it possible to consider the number of structural domains as a reliable indicator of a microorganism’s affiliation with a particular division and to judge about “direction” of their evolution. The findings allow us to consider the loops and repeats in these proteins as unique imprints of molecular evolution.
Similar content being viewed by others
Abbreviations
- EF-1:
-
elongation factor 1 (EF-1 in this work is interpreted as elongation factor EF-Tu and EF-Ts for eubacteria, aEF-1 for archaea, and EEF-1 for eukaryotes)
- EF-2:
-
elongation factor 2 (EF-2 in this work is interpreted as elongation factor EF-G for eubacteria, aEF-2 for archaea, and EEF-2 for eukaryotes)
- G-family:
-
the GTP-binding protein family (GTP)
- PNPase:
-
polynucleotide phosphorylase
References
Lemieux, U., and Spohr, U. (1994) Adv. Carbohydrate Chem. Biochem., 50, 1–20.
Karush, F. (1950) J. Am. Chem. Soc., 72, 2705–2713.
Blake, C., Koenig, D., Mair, G., North, A., Phillips, D., and Sarma, V. (1965) Nature, 206, 757–761.
McDonald, R., Steitz, T., and Engelman, D. (1979) Biochemistry, 18, 338–342.
Kjeldgaard, M., Nissen, P., Thirup, S., and Nyborg, J. (1993) Structure, 1, 35–50.
Dobson, C. (1999) Trends Biochem. Sci., 24, 329–332.
Pedersen, J., Andersen, C., and Otzen, D. (2010) FEBS J., 277, 4591–4601.
Uversky, A., Gillspie, J., and Fink, A. (2000) Proteins, 41, 415–427.
Serdyuk, I. N. (2007) Mol. Biol. (Moscow), 42, 287–313.
Murzin, A. (1993) The EMBO J., 12, 861–867.
Mosavi, L., Cammett, T., Desrosiers, D., and Peng, Z. (2004) Protein Sci., 13, 1435–1448.
Betz, S. (1993) Protein Sci., 2, 1551–1558.
Fraser, R., MacRae, T., and Suzuki, E. (1979) J. Mol. Biol., 129, 463–448.
Daley, D. (2008) Curr. Opin. Struct. Biol., 18, 420–424.
Timasheff, S., and Gorbunoff, M. (1967) Annu. Rev. Biochem., 36, 13–54.
Dunker, A., and Lawson, J. (2001) J. Mol. Graphics Model., 19, 26–59.
Dunker, A., Brown, C., and Lawson, J. (2002) Biochemistry, 41, 6573–6582.
Ptitsyn, O. (1995) Adv. Protein Chem., 47, 183–229.
Receveur-Brechot, V., Bourish, J., and Uversky, V. (2006) Proteins, 62, 24–45.
Jeffry, C. (1999) TIBS, 24, 8–11.
Budkevich, T., Timchenko, A., and Tiktopulo, E. (2002) Biochemistry, 41, 15342–15349.
Deryusheva, E. I., Galzitskaya, O. V., and Serdyuk, I. N. (2008) Mol. Biol. (Moscow), 42, 1067–1078.
Deryusheva, E. I., Levin, D. M., and Serdyuk, I. N. (2009) Izv. TulGU. Estestv. Nauki, 3, 229–237.
Evarsson, A., Brazhnikov, E., and Garber, M. (1994) The EMBO J., 13, 3669–3677.
McGinness, K., and Sauer, R. (2004) PNAS, 101, 13454–13459.
Dahlberg, A., and Dahlberg, J. (1975) Proc. Natl. Acad. Sci. USA, 72, 2940–2944.
Blumenthal, T., Young, R., and Brown, S. (1976) J. Biol. Chem., 251, 2740–2743.
Regnier, P., Grunberg-Manago, M., and Portier, C. (1987) J. Biol. Chem., 262, 63–68.
Subramanian, A. (1983) Prog. Nucleic Acid Res. Mol. Biol., 28, 101–142.
Deryusheva, E. I., Machulin, A. V., Selivanova, O. M., and Serdyuk, I. N. (2010) Mol. Biol. (Moscow), 44, 728–734.
Bycroft, M., Hubbard, T., Proctor, M., Freud, S., and Murzin, G. (1997) Cell, 24, 235–242.
Kim, D., Chivian, D., and Baker, D. (2004) Nucleic Acids Res., 32, W526–W531.
Frank, J. (2001) Proc. Natl. Acad. Sci. USA, 98, 11991–11996.
Fink, A. (2005) Curr. Opin. Struct. Biol., 15, 35–41.
Dyson, H., and Wright, P. (2005) Nat. Rev. Mol. Cell Biol., 6, 197–208.
Tompa, P. (2002) Trends Biochem. Sci., 27, 527–533.
Romero, P., Obradovic, Z., Li, X., Garner, E., Brown, C., and Dunker, A. (2001) Proteins, 42, 38–48.
Yang, Z., Thomson, R., McNeil, P., and Esnouf, R. (2005) Bioinformatics, 21, 3369–3376.
Linding, R., Jensen, L., Diella, F., Bork, P., Gibson, T., and Russell, R. (2003) Structure, 11, 1453–1459.
Coeytaux, K., and Poupon, A. (2005) Bioinformatics, 21, 1891–1900.
Dosztanyi, Z., Csizmok, V., Tompa, P., and Simon, I. (2005) Bioinformatics, 21, 3433–3434.
Linding, R., Russell, R., Neduva, V., and Gibson, T. (2003) Nucleic Acids Res., 31, 3701–3708.
Prilusky, J., Felder, C., Zeev-Ben-Mordehai, T., Rydberg, E., Man, O., Beckmann, J., Silman, I., and Sussman, J. (2005) Bioinformatics, 21, 3435–3438.
Galzitskaya, O. V., Garbuzinsky, S. A., and Lobanov, M. Yu. (2006) Mol. Biol. (Moscow), 40, 341–348.
Galzitskaya, O., Garbuzynskiy, S., and Lobanov, M. (2006) Bioinformatics, 22, 2948–2949.
Andersen, G., Pedersen, L., and Valente, L. (2000) Mol. Cell, 6, 1261–1266.
Steenkamp, E., Wright, J., and Baldauf, S. (2005) Mol. Biol. Evol., 23, 93–106.
Galzitskaya, O., Deryusheva, E., and Serdyuk, I. (2008) J. Comput. Sci. Syst. Biol., 1, 73–80.
Kjeldgard, M., Nissen, P., Thirup, S., and Nyborg, J. (1993) Structure, 1, 35–50.
Kjeldgard, M., and Nyborg, J. (1992) J. Mol. Biol., 223, 721–742.
Schultz, J., Milpetz, F., Bork, P., and Ponting, C. (1998) Proc. Natl. Acad. Sci. USA, 95, 5857–5864.
Sonnhammer, E., Eddy, S., Birney, E., Bateman, A., and Durbin, R. (1998) Nucleic Acids Res., 26, 320–322.
Hulo, N., Bairoch, A., Bulliard, V., Cerutti, L., Cuche, B., Castro, E., Lachaize1, C., Langendijk-Genevaux, P., and Sigrist, C. (2008) Nucleic Acids Res., 36, 245–249.
Bairoch, A., and Apweiler, R. (2000) Nucleic Acids Res., 28, 45–48.
Bryson, K., McGuffin, L., Marsden, R., Ward, J., Sodhi, J., and Jones, D. (2005) Nucleic Acids Res., 33, 36–38.
Guttel, R., Larsen, N., and Woese, C. (1994) Microbiol. Rev., 58, 10–26.
Doolittle, W. (1999) Science, 284, 2124–2128.
Lartigue, C., Glass, J., Alperovich, N., Pieper, R., Parmar, P., Hutchison, C., Smith, H., and Venter, J. (2007) Science, 317, 632–638.
Dagan, T., Artzy-Randrup, Y., and Martin, W. (2008) Proc. Natl. Acad. Sci. USA, 105, 10039–10044.
Bhugra, B. (1992) Mol. Microbiol., 6, 1149–1154.
Neimark, H. C. (1979) Phylogenetic Relations between Mycoplasmas and Other Prokaryotes. The Mycoplasmas (Barile, M. F., and Razin, S., eds.) New York, Vol. 1, pp. 43–61.
Borkhsenius, S. N. (2000) Mycoplasms. Molecular and Cell Biology, Interaction with Mammalian Immune System, Pathogenicity, Diagnostics [in Russian], Nauka, Moscow.
Blanchard, A. (1990) Mol. Microbiol., 4, 669–676.
Amblar, M., Barbas, A., Gomez-Puertas, P., and Arraiano, C. (2007) RNA, 13, 317–327.
Boni, I., Artamonova, V., and Dreyfus, M. (2000) J. Bacteriol., 182, 5872–5879.
De Boer, P., Vos, H., Faber, A., Vos, J., and Raue, H. (2006) RNA, 12, 263–271.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E. I. Deryusheva, O. M. Selivanova, I. N. Serdyuk, 2012, published in Uspekhi Biologicheskoi Khimii, 2012, Vol. 52, pp. 177–202.
Rights and permissions
About this article
Cite this article
Deryusheva, E.I., Selivanova, O.M. & Serdyuk, I.N. Loops and repeats in proteins as footprints of molecular evolution. Biochemistry Moscow 77, 1487–1499 (2012). https://doi.org/10.1134/S000629791213007X
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629791213007X