Abstract
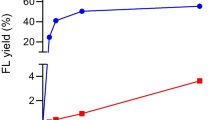
O-GlcNAcase (OGA) is a family 84 glycoside hydrolase catalyzing the hydrolytic cleavage of O-linked β-N-acetylglucosamine (O-GlcNAc) from serine and threonine residues of proteins. Thus far, three forms of OGA have been identified in humans. Here we optimized the expression of these isoforms in E. coli and characterized their kinetic properties. Using Geno 3D, we predicted that N-terminal amino acids 63–342 form the catalytic site for O-GlcNAc removal and characterized it. Large differences are observed in the Km value and catalytic efficiency (kcat/Km) for the three OGA variants, though all of them displayed O-GlcNAc hydrolase activity. The full-length OGA had the lowest Km value of 0.26 mM and the highest catalytic efficiency of 3.51·103. These results reveal that the N-terminal region (a.a. 1–350) of OGA contains the catalytic site for glycoside hydrolase and the C-terminal region of the coding sequence has the ability to stabilize the native three-dimensional structure and further affect substrate affinity.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- fOGA:
-
full-length O-GlcNAcase
- IPTG:
-
isopropyl-L-thio-β-D-galactopyranoside
- LB:
-
Luria-Bertani (broth)
- MGEA5:
-
meningioma expressed antigen 5
- 4-MU-GlcNAc:
-
4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-glucopyranoside
- OGA:
-
O-GlcNAcase
- PCD:
-
programmed cell death
- sOGA:
-
the shortest OGA
- vOGA:
-
variant of OGA
References
Henrissat, B., and Bairoch, A. (1996) Biochem. J., 316, 695–696.
Wells, H., Vosseller, K., and Hart, G. W. (2001) Science, 291, 2376–2378.
Torres, C. R., and Hart, G. W. (1984) J. Biol. Chem., 259, 3308–3317.
Hart, G. W., Housley, M. P., and Slawson, C. (2007) Nature, 446, 1017–1022.
Zeidan, Q., and Hart, G. W. (2010) J. Cell. Sci., 123, 13–22.
Zhang, F., Su, K., Yang, X., Bowe, D. B., Paterson, A. J., and Kudlow, J. E. (2003) Cell, 115, 715–725.
Yang, X., Ongusaha, P. P., Miles, P. D., Havstad, J. C., Zhang, F., So, W. V., Kudlow, J. E., Michell, R. H., Olefsky, J. M., and Field, S. J. (2008) Nature, 451, 964–969.
Fischer, P. M. (2008) Nat. Chem. Biol., 4, 448–449.
Kang, J. G., Park, S. Y., Ji, S., Jang, I., Park, S., and Kim, H. S. (2009) J. Biol. Chem., 284, 34777–34784.
Lubas, W. A., Frank, D. W., Krause, M., and Hanover, J. A. (1997) J. Biol. Chem., 272, 9316–9324.
Gao, Y., Wells, L., Comer, F. I., Parker, G. J., and Hart, G. W. (2001) J. Biol. Chem., 276, 9838–9845.
Bertram, L., Blacker, D., Mullin, K., Keeney, D., Jones, J., Basu, S., Yhu, S., and Tanzi, R. E. (2000) Science, 290, 2302–2303.
Schultz, J., and Pils, B. (2002) FEBS Lett., 529, 179–182.
Comtesse, N., Maldener, E., and Meese, E. (2001) Biochem. Biophys. Res. Commun., 283, 634–640.
Wells, L., Gao, Y., Mahoney, J. A., Vosseller, K., Chen, C., Rosen, A., and Hart, G. W. (2002) J. Biol. Chem., 277, 1755–1761.
Bradford, M. M. (1976) Anal. Biochem., 72, 248–254.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Christophe, C., Martin, J., Gilbert, D., and Christophe, G. (2002) Bioinformatics, 18, 213–214.
Macauley, M. S., Whitworth, G. E., Debowski, A. W., Chin, D., and Vocadlo, D. J. (2005) J. Biol. Chem., 280, 25313–25322.
Kim, E. J., Kang, D. O., Love, D. C., and Hanover, J. A. (2006) Carbohydr. Res., 341, 971–982.
Cuetinbasu, N., Macauley, M. S., Stubbs, K. A., Drapala, R., and Vocadlo, D. J. (2006) Biochemistry, 45, 3835–3844.
Dennis, R. J., Taylor, E. J., Macauley, M. S., Stubbs, K. A., Turkenburg, J. P., Hart, S. J., Black, G. N., Vocadlo, D. J., and Davies, G. J. (2006) Nat. Chem. Biol., 13365–13371.
Yin, J., Li, L., Shaw, N., Li, Y., Song, J. K., Zhang, W., Xia, C. F., Zhang, R. G., Joachimiak, A., Zhang, H. C., Wang, L. X., Liu, Z. J., and Wang, P. (2009) PLoS ONE, 4, e4658.
Zachara, N. E., and Hart, G. W. (2004) Biochim. Biophys. Acta, 1673, 13–28.
Author information
Authors and Affiliations
Corresponding authors
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1134/S0006297910100160
Rights and permissions
About this article
Cite this article
Li, J., Huang, Cl., Zhang, Lw. et al. Isoforms of human O-GlcNAcase show distinct catalytic efficiencies. Biochemistry Moscow 75, 938–943 (2010). https://doi.org/10.1134/S0006297910070175
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297910070175