Abstract
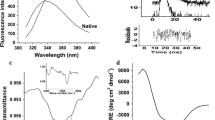
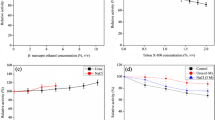
The biophysical properties of Bacillus kaustophilus leucyl aminopeptidase (BkLAP) were examined in terms of analytical ultracentrifugation, fluorescence spectroscopy, and circular dichroism. By using the analytical ultracentrifuge, we demonstrated that tetrameric BkLAP exists as the major form in solution at protein concentration of 1.5 mg/ml at pH 8.0. The native enzyme started to unfold beyond ∼1 M GdnHCl and reached an unfolded intermediate with [GdnHCl]1/2 at 1.8 M. Thermal unfolding of BkLAP was found to be highly irreversible and led to a marked formation of aggregates.
Similar content being viewed by others
Abbreviations
- BkLAP:
-
B. kaustophilus LAP
- CD:
-
circular dichroism
- GdnHCl:
-
guanidine hydrochloride
- LAP:
-
leucyl aminopeptidase
- L-Leu-p-NA:
-
L-leucine-p-nitroanilide
- Ni2+-NTA:
-
nickel nitrilotriacetate
- p-NA:
-
p-nitroaniline
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Taylor, A. (1993) FASEB J., 7, 290–298.
Matsui, M., Fowler, J. H., and Walling, L. L. (2006) Biol. Chem., 387, 1535–1544.
Burley, S. K., David, P. R., Taylor, A., and Lipscomb, W. (1990) Proc. Natl. Acad. Sci. USA, 87, 6878–6882.
Strater, N., Sherrat, D. J., and Collons, S. D. (1999) EMBO J., 18, 4513–4522.
Vogt, V. M. (1970) J. Biol. Chem., 245, 4760–4769.
Charlier, D., Kholti, A., Huysveld, N., Gigot, D., Maes, D., Thia-Toong, T. L., and Glansdorff, N. (2000) J. Mol. Biol., 302, 411–426.
Chao, W. S., Gu, Y. Q., Pautot, V., Bray, E. A., and Walling, L. L. (1999) Plant Physiol., 120, 979–992.
Beninga, J., Rock, K. L., and Goldberg, A. L. (1998) J. Biol. Chem., 273, 18534–18542.
Daggett, M., and Fersht, A. R. (2003) Trends Biochem. Sci., 28, 18–25.
Nolting, B., Golbik, R., and Fersht, A. R. (1995) Proc. Natl. Acad. Sci. USA, 92, 10668–10672.
Plaxco, K. W., and Dobson, C. M. (1996) Curr. Opin. Struct. Biol., 6, 630–636.
Panda, K. W., Gorovits, B. M., and Horowitz, P. M. (2000) J. Biol. Chem., 275, 63–70.
Screerama, N., and Woody, K. W. (2004) Meth. Enzymol., 383, 318–351.
Lin, L. L., Hsu, W. H., Wu, C. P., Chi, M. C., Chou, W. M., and Hu, H. Y. (2004) Extremophiles, 8, 79–87.
Chi, M. C., Huang, H. B., Liu, J. S., Wang, W. C., Liang, W. C., and Lin, L. L. (2006) FEMS Microbiol. Lett., 260, 156–161.
Chi, M. C., Liu, J. S., Wang, W. C., Lin, L. L., and Huang, H. B. (2008) Biochimie, 90, 811–819.
Chi, M. C., Ong, P. L, Hsu, W. H., Chen, Y. H., Huang, H. B., and Lin, L. L. (2008) Int. J. Biol. Macromol., 43, 481–487.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Brown, P. H., and Schuck, P. (2006) Biophys. J., 90, 4651–4661.
Lunn, F. A., MacLeod, T. J., and Bearne, S. L. (2008) Biochem. J., 412, 113–121.
Loveridge, E. J., Rodriguez, R. J., Swanwick, R. S., and Allemann, R. K. (2009) Biochemistry, 48, 5822–5933.
Nakagawa, Y., Saburi, W., Takada, M., Hatada, Y., and Horikoshi, K. (2008) Biochim. Biophys. Acta, 1784, 2004–2011.
Gu, Y. Q., Pautot, V., Holzer, F. M., and Walling, L. L. (1996) Plant Physiol., 110, 1257–1266.
Gu, Y. Q., Holzer, F. M., and Walling, L. L. (1999) Eur. J. Biochem., 263, 726–735.
Herrera-Camacho, I., Rosas-Murrieta, N. H., Rolo-Domingguez, A., Millan, L., Reyes-Leyva, J., Santos-Lopez, G., and Suarez-Rendueles, P. (2007) FEBS J., 274, 6228–6240.
Gallagher, S. R. (2001) in Curr. Protoc. Protein Sci., Chap. 6, Unit 6.5, John Wiley & Sons, New York.
Shriver, J. W., and Edmondson, S. P. (2009) Meth. Mol. Biol., 490, 57–82.
Gruber, C. W., Cemazar, M., Mechler, A., Martin, L. L., and Crail, D. J. (2009) Peptide Sci., 92, 35–43.
Dengra-Pozo, J., Martinez-Rodriguez, S., Contreras, L. M., Prieto, J., Andujar-Sanchez, M., Clemente-Jimenez, J. M., Las Heras-Vazquez, F. J., Rodriguez-Vico, F., and Neira, J. L. (2009) Biopolymers, 91, 757–772.
Freire, E., van Osdol, W. W., Mayorga, O. L., and Sanchez-Ruiz, J. M. (1990) Annu. Rev. Biophys. Biophys. Chem., 19, 159–188.
Sanchez-Ruiz, J. M. (1992) Biophys. J., 61, 921–935.
Tello-Solis, S. R., and Hernandez-Arana, A. (1995) Biochem. J., 311, 969–974.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, MC., Chang, HP., Chang, GG. et al. Biophysical characterization of a recombinant leucyl aminopeptidase from Bacillus kaustophilus . Biochemistry Moscow 75, 642–647 (2010). https://doi.org/10.1134/S0006297910050159
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297910050159