Abstract
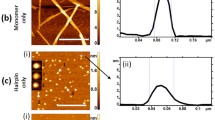
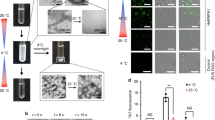
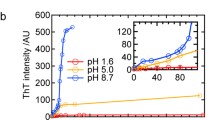
The sequence-reversed form of a small heat shock protein, HSP12.6 (retro-HSP12.6), has been reported to fold and assemble into structured tetramers in aqueous solution. Upon raising the protein concentration to ∼1.0–1.5 mg/ml, tetrameric retro-HSP12.6 is known to display a tendency to associate further into spherical beads of 18–20 nm in diameter containing folded protein subunits. Here we report that storage of this protein at low temperatures leads to further association of the beaded structures into linear and ring-shaped amyloid nanofibers of 18–20 nm in diameter. The electron micrographs presented in this communication provide the best visual evidence yet that amyloids can form through the association of smaller structured bead-like intermediates. The results also suggest that folded β-sheet-rich subunits can participate in amyloid formation.
Similar content being viewed by others
References
Rousseau, F., Schymkowitz, J., and Serrano, L. (2006) Curr. Opin. Struct. Biol., 16, 118–126.
Guptasarma, P. (1992) FEBS Lett., 310, 205–210.
Guptasarma, P. (1996) Trends Biotechnol., 14, 42–43.
Shukla, A., Raje, M., and Guptasarma, P. (2003) J. Biol. Chem., 278, 26505–26510.
Shukla, A., Raje, M., and Guptasarma, P. (2003) Protein Eng., 16, 875–879.
Malisauskas, M., Zamotin, V., Jass, J., Noppe, W., Dobson, C. M., and Morozova-Roche, L. A. (2003) J. Mol. Biol., 330, 879–890.
Kelly, J. W. (1998) Curr. Opin. Struct. Biol., 8, 101–106.
Dobson, C. M. (2001) Philos. Trans. R. Soc. Lond. B. Biol. Sci., 356, 133–145.
Chiti, F., Webster, P., Taddei, N., Clark, A., Stefani, M., Ramponi, G., and Dobson, C. M. (1999) Proc. Natl. Acad. Sci. USA, 96, 3590–3594.
Prusiner, S. B., Scott, M. R., de Armond, S. J., and Cohen, F. E. (1998) Cell, 93, 337–345.
Janowski, R., Kozak, M., Jankowska, E., Grzonka, Z., Grubb, A., Abrahamson, M., and Jaskolski, M. (2001) Nat. Struct. Biol., 8, 316–320.
Knaus, K. J., Morillas, M., Swietnicki, W., Malone, M., Surewicz, W. K., and Yee, V. C. (2001) Nat. Struct. Biol., 8, 770–774.
Staniforth, R. A., Giannini, S., Higgins, L. D., Conroy, M. J., Hounslow, A. M., Jerala, R., Craven, C. J., and Waltho, J. P. (2001) EMBO J., 20, 4774–4781.
Jimenez, J. L., Guijarro, J. I., Orlova, E., Zurdo, J., Dobson, C. M., Sunde, M., and Saibil, H. R. (1999) EMBO J., 18, 815–821.
Sinha, N., Tsai, C. J., and Nussinov, R. (2001) Protein Eng., 14, 93–103.
Siepen, J. A., Radford, S. E., and Westhead, D. R. (2003) Protein Sci., 12, 2348–2359.
Blackley, H. K., Patel, N., Davies, M. C., Roberts, C. J., Tendler, S. J., Wilkinson, M. J., and Williams, P. M. (1999) Exp. Neurol., 158, 437–443.
Carrotta, R., Bauer, R., Waninge, R., and Rischel, C. (2001) Protein Sci., 10, 1312–1328.
Lee, H. J., and Lee, S. J. (2002) J. Biol. Chem., 277, 48976–48983.
Gorman, P. M., Yip, C. M., Fraser, P. E., and Chakrabarty, A. (2003) J. Mol. Biol., 325, 743–757.
Srinivasan, R., Jones, E. M., Liu, K., Ghiso, J., Marchant, R. E., and Zagorski, M. G. (2003) J. Mol. Biol., 333, 1003–1023.
Shahi, P., Sharma, R., Sanger, S., Kumar, I., and Jolly, R. S. (2007) Biochemistry, 46, 7365–7373.
Lundberg, K. M., Stenland, C. J., Cohen, F. E., Prusiner, S. B., and Millhauser, G. L. (1997) Chem. Biol., 4, 345–355.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2008, Vol. 73, No. 6, pp. 848–853.
Rights and permissions
About this article
Cite this article
Shukla, A., Raje, M. & Guptasarma, P. Coalescence of spherical beads of retro-HSP12.6 into linear and ring-shaped amyloid nanofibers. Biochemistry Moscow 73, 681–685 (2008). https://doi.org/10.1134/S0006297908060084
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297908060084