Abstract
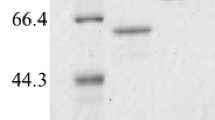
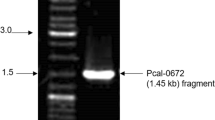
The aim of this study was to isolate a novel amylomaltase gene from community DNA of soil samples collected from Ban Nong Khrok hot spring in Thailand without bacterial cultivation. Using PCR, a 1.5 kb full-length gene was amplified and ligated with pGEM®-T easy vector to transform into Escherichia coli DH5 α for sequencing. The obtained gene encoding an amylomaltase consisted of 1,503 bp that translated into 500 amino acids. Amino acid sequence deduced from this gene was highly homologous with that of amylomaltase from Thermus thermophillus ATCC 33923. In order to express the enzyme, the cloned gene was subcloned into plasmid pET-17b and introduced into E. coli BL21(DE3). The maximum expression was observed when the cloned cells were cultured at 37°C for 6 h with 0.5 mM IPTG induction. By 10% SDS-PAGE, the relative molecular mass of the purified amylomaltase was approximately 58 kDa. This enzyme was optimally active at 70°C and pH 9.0. In addition, the enzyme could hydrolyze pea starch to yield the largering cyclodextrins with degrees of polymerization of 23 and higher. It is noted that CD29 was the product in the largest quantity under all tested conditions.
Similar content being viewed by others
References
Takaha, T. and Smith, S.M., Biotechnol. Genet. Eng. Rev., 1999, vol. 16, pp. 257–280.
Bhuiyan, S.H., Kitaoka, M., and Hayashi, K., J. Mol. Catal. B: Enzym., 2003, vol. 22, nos. 1–2, pp. 45–53.
Terada, Y., Fujii, K., Takaha, T., and Okada, S., Appl. Environ. Microbiol., 1999, vol. 65, no. 3, pp. 910–915.
Endo, T., Zheng, M., and Zimmermann, W., Aust. J. Chem., 2002, vol. 55, nos. 1–2, pp. 39–48.
Larsen, K.L., J. Incl. Phenom. Macro, 2002, vol. 43, nos. 1–2, pp. 1–13.
Kaulpiboon, J., Pongsawasdi, P., and Zimmermann, W., J. Mol. Recognit., 2010, vol. 23, no. 5, pp. 480–485.
Machida, S., Ogawa, S., Xiaohua, S., Takaha, T., Fujii, K., and Hayashi, K., FEBS Lett., 2000, vol. 486, no. 2, pp. 131–135.
Monod, J. and Torriani, A.M., Ann. Inst. Oasteur (Paris), 1950, vol. 78, no. 1, pp. 65–77.
Stassi, D.L., Lopez, P., Espinosa, M., and Lacks, S.A., Proc. Natl. Acad. Sci. USA, 1981, vol. 78, no. 11, pp. 7028–7032.
Pugsley, A.P. and Dubreuil, C., Mol. Microbiol., 1988, vol. 2, no. 4, pp. 473–479.
Goda, S.K., Eissa, O., Akhtar, M., and Minton, N.P., Microbiology, 1997, vol. 143, no. 10, pp. 3287–3294.
Jeon, B.S., Taguchi, H., Sakai, H., Ohshima, T., Wakagi, T., and Matsuzawa, H., Eur. J. Biochem., 1997, vol. 248, no. 1, pp. 171–178.
Kaper, T., Talik, B., Ettema, T.J., Bos, H., van der Maarel, M.J., and Dijkhuizen, L., Appl. Environ. Microbiol., 2005, vol. 71, no. 9, pp. 5098–5106.
Daniel, R., Curr. Opin. Biotechnol., 2004, vol. 15, no. 3, pp. 199–204.
Hayashi, H., Abe, T., Sakamoto, M., Ohara, H., Ikemura, T., Sakka, K., and Benno, Y., Can. J. Microbiol., 2005, vol. 51, no. 3, pp. 251–259.
Tang, K., Utairungsee, T., Kanokratana, P., Sriprang, R., Champreda, V., Eurwilaichitr, L., and Tanapongpipat, S., FEMS Microbiol. Lett., 2006, vol. 260, no. 1, pp. 91–99.
Maniatis, T., Fritsch, E.F., and Sambrook, J., Molecular Cloning: a Laboratory Manual, New York: Cold Spring Habor, 1982.
Sawadee, K., Expression and Characterization of Amylomaltase Gene Cloned Directly from Soil Bacterial DNA, Bangkok: Thammasat Univ., 2011.
Weber, K. and Osborn, M., Proteins and SDS: Molecular Weight Determination on Polyacrylamide Gels and Related Procedures, Neurath, H., Hill, R.L., and Border, C., Eds., New York: The Protein Academic Press, 1975, pp. 179–233.
Park, J.H., Kim, H.J., Kim, Y.H., Cha, H., Kim, Y.W., Kim, T.J., Kim, Y.R., and Park, K.H., Carbohydr. Res., 2007, vol. 67, no. 2, pp. 164–173.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, nos. 1–2, pp. 248–254.
Lee, B.H., Oh, D.K., and Yoo, S.H., New Biotechnol., 2009, vol. 26, nos. 1–2, pp. 29–36.
Xavier, K.B., Peist, R., Kossmann, M., Boos, W., and Santos, H., J. Bacteriol., 1999, vol. 181, no. 11, pp. 3358–3367.
Kitahata, S., Murakami, H., and Okada, S., Agr. Biol. Chem., 1989, vol. 53, no. 7, pp. 2653–2659.
Srisimarat, W., Powviriyakul, A., Kaulpiboon, J., Krusong, K., and Zimmermann, W., Pongsawasdi, P., J. Incl. Phenom. Macrocycl. Chem., 2010, vol. 70, nos 3–4, pp. 369–375.
Schmidt, J. and John, M., Biochim. Biophys. Acta, 1979, vol. 566, no. 1, pp. 100–114.
Feil, R., Gabelsberger, J., Kellermann, J., and Schleifer, K.H., Eur. J. Biochem., 1992, vol. 207, no. 1, pp. 81–88.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Prikladnaya Biokhimiya i Mikrobiologiya, 2014, Vol. 50, No. 1, pp. 25–33.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sawasdee, K., Rudeekulthamrong, P., Zimmermann, W. et al. Direct cloning of gene encoding a novel amylomaltase from soil bacterial DNA for large-ring cyclodextrin production. Appl Biochem Microbiol 50, 17–24 (2014). https://doi.org/10.1134/S000368381306015X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000368381306015X