Abstract
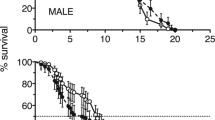
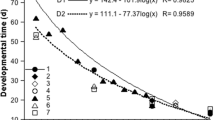
Salinity tolerance, energy metabolism, buoyancy, and passive sinking and swimming speeds have been studied for comparative assessment of the adaptive potential of two species of cyclopoid copepods in the Black Sea, the native Oithona similis and new invader Oithona davisae. Both species were considered marine euryhaline copepods, but the range of salinity tolerance of O. davisae was much broader (5–55‰). The energy metabolism, locomotor activity, mean body mass density, and speed of passive sinking at the same temperature were significantly higher in O. davisae than in O. similis. The relationship between the physiological and behavioral parameters and ecological characteristics of the species are discussed.
Similar content being viewed by others
References
D. A. Altukhov and A. D. Gubanova, “Oithona brevicornis Giesbrecht in Sevastopol Bay in October 2005–March 2006,” Morsk. Ekol. Zh. 5 (2), 32 (2006).
V. G. Dvoretskii and A. G. Dvoretskii, Biology and Role of Oithona similis in Zooplankton of the Arctic Seas, Ed. by P. R. Makarevich (Karelian Scientific Center, Russian Academy of Sciences, Apatity, 2011) [in Russian].
Yu. A. Zagorodnyaya, “Oithona brevicornis in Sevastopol Bay: fortuity or new invader in the Black Sea,” Ekol. Morya 61, 43 (2002).
A. V. Kovalev, “Survival of some pelagic copepods from the Black and Mediterranean seas in the water with different salinity,” Gidrobiol. Zh. 11 (1), 43–48 (1966).
A. V. Kovalev, “Size composition of planktonic Copepoda at the different depths of the Black Sea,” Gidrobiol. Zh. 3 (3), 74–77 (1967).
A. V. Kovalev, “Why Oithona nana Giesbr. disappeared in the Black Sea plankton in the end of 80’s of 20 century?” Morsk. Ekol. Zh. 6 (1), 43 (2007).
A. V. Kovalev, V. E. Zaika, N. A. Ostrovskaya, et al., “Mnemiopsis mccradyi Mayer, 1900 is a new habitant of the Black Sea,” Gidrobiol. Zh. 30 (3), 104–107 (1994).
Zh. P. Selifonova, “Oithona brevicornis Giesbrecht (Copepoda: Cyclopoida) in zooplankton of the ports of northeast shelf of the Black Sea,” Biol. Vnutr. Vod, No. 1, 33–35 (2009).
D. Atienza, A. Calbet, E. Saiz, et al., “Trophic impact, metabolism, and biogeochemical role of the marine cladoceran Penilia avirostris and the co-dominant copepod Oithona nana in NW Mediterranean coastal waters,” Mar. Biol. 150, 221–235 (2006).
R. Beltrão, M. Monde, and H. Ueda, “Characteristics and regional classification of the copepod community in Ariake Bay with note on comparison with three decades ago,” J. Oceanogr. 67 (1), 47–58 (2011).
C. Castellani, C. Robinson, T. Smith, and R. S. Lampitt, “Temperature affects respiration rate of Oithona similis,” Mar. Ecol.: Progr. Ser. 285, 129–135 (2005).
F. Ferrari and J. Orsi, “Oithona davisae, new species, and Limnoithona sinensis (Burkckhard, 1912) (Copepoda: Oithonidae) from the Sacramento–San Joaquin estuary, California,” J. Crustacean Biol. 4 (1), 106–126 (1984).
F. C. Hansen, C. Mollmann, U. Schutz, and H.-H. Hinrichsen “Spatio-temporal distribution of Oithona similis in the Bornholm Basin (Central Baltic Sea),” J. Plankton Res. 26 (6), 659–668 (2004).
K. Hirakawa, “New records of the North Pacific coastal planktonic copepods, Acartia omorii (Acartiidae) and Oithona davisae (Oithonidae) from southern Chile,” Bull. Mar. Sci. 42, 337–339 (1988).
J. Hiromi, T. Nagata, and S. Kadota, “Respiration of the small planktonic copepod Oithona davisae at different temperatures,” Bull. Plankton Soc. Jpn. 35, 143–148 (1988).
T. Ikeda, Y. Kanno, K. Ozaki, and A. Shinada, “Metabolic rates of epipelagic marine copepods as a function of body mass and temperature,” Mar. Biol. 139, 587–596 (2001).
T. Kiørboe and A. W. Visser, “Predator and prey perception in copepods due to hydromechanical signals,” Mar. Ecol.: Progr. Ser. 179, 81–95 (1999).
L. A. Lougee, S. M. Bollens, and S. R. Avent, “The effects of haloclines on the vertical distribution and migration of zooplankton,” J. Exp. Mar. Biol. Ecol. 278, 111–134 (2002).
V. Mihneva and K. Stefanova, “The non-native copepod Oithona davisae (Ferrari F.D. and Orsi, 1984) in the Western Black Sea: seasonal and annual abundance variability,” BioInvasions Rec. 2 (2), 119–124 (2013).
M. Miloslavic, D. Lucic, J. Njire, et al., “Zooplankton composition and distribution across coastal and offshore waters off Albania (Southern Adriatic) in late spring,” Acta Adriat. 53 (2), 165–180 (2012).
Y. Nakamura and J. T. Turner, “Predation and respiration by the small cyclopoid copepod Oithona similis. How important is feeding on ciliates and heterotrophic flagellates?” J. Plankton Res. 19 (9), 1275–1288 (1997).
K. Nakata and T. Nakane, “Respiration of plankton in the Mikawa Bay,” Pollut. Control. 22, 281–294 (1987).
T. G. Nielsen, E. F. Møller, S. Satapoomin, et al., “Egg hatching rate of the cyclopoid copepod Oithona similis in arctic and temperate waters,” Mar. Ecol.: Progr. Ser. 236, 301–306 (2002).
G. A. Paffenhöfer, “On the ecology of marine cyclopoid copepods (Crustacea, Copepoda),” J. Plankton Res. 15, 37–55 (1993).
E. Saiz, A. Calbet, E. Broglio, and P. Mari, “Effects of small-scale turbulence on copepods: the case of Oithona davisae,” Limnol. Oceanogr. 48 (3), 1304–1311 (2003).
C. Svensen and T. Kiørboe, “Remote prey detection in Oithona similis: hydromechanical versus chemical cues,” J. Plankton Res. 22 (6), 1155–1166 (2000).
L. S. Svetlichny, E. S. Hubareva, and E. G. Arashkevich, “Physiological and behavioral response to hypoxia in active and diapausing copepodites Stage V Calanus euxinus,” Adv. Limnol. 52, special issue, 507–519 (1998).
L. S. Svetlichny, E. S. Hubareva, F. Erkan, and A. G. Gucu, “Physiological and behavioral aspects of Calanus euxinus females (Copepoda, Calanoida) during vertical migration,” Mar. Biol. 137, 963–971 (2000).
L. Svetlichny, E. Hubareva, and A. Khanaychenko, “Calanipeda aquae dulcis and Arctodiaptomus salinus: an exceptionally euryhaline osmoconformers as evident from mortality, oxygen consumption, and mass density patterns,” Mar. Ecol.: Progr. Ser. 470, 15–29 (2012a).
L. Svetlichny, A. Khanaychenko, E. Hubareva, and L. Aganesova, “Partitioning of respiratory energy and environmental tolerance in Calanipeda aquaedulcis and Arctodiaptomus salinus,” Estuarine, Coastal Shelf Sci. 114, 199–207 (2012b).
A. Temnykh and Sh. Nishida, “New record of the planktonic copepod Oithona davisae Ferrari and Orsi in the Black Sea with notes on the identity of Oithona brevicornis,” Aquat. Invasions 7, 425–431 (2012).
J. T. Turner, “The importance of small planktonic copepods and their roles in pelagic marine food webs,” Zool. Stud. 43 (2), 255–266 (2004).
M. Uchima and R. Hirano, “Swimming behavior of the marine copepod Oithona davisae: internal control and search for environment,” Mar. Biol. 99, 47–56 (1988).
S.-I. Uye and K. Sano, “Seasonal reproductive biology of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet,” Mar. Ecol.: Progr. Ser. 118, 121–128 (1995).
B. Wend-Heckmann, Dissertation in Natural Sciences (Am Fachbereich Biologie/Chemie der Universität Bremen, Germany, 2013).
O. A. Yunev, S. Moncheva, and J. Carstensen, “Longterm variability of vertical chlorophyll a and nitrate profiles in the open Black Sea: eutrophication and climate change,” Mar. Ecol.: Progr. Ser. 294, 95–107 (2005).
S. Zamora-Terol and E. Saiz, “Effects of food concentration on egg production and feeding rates of the cyclopoid copepod Oithona davisae,” Limnol. Oceanogr. 58 (1), 376–387 (2013).
S. Zamora-Terol, T. G. Nielsen, and E. Saiz, “Plankton community structure and role of Oithona similis on the western coast of Greenland during the winterspring transition,” Mar. Ecol.: Progr. Ser. 483, 85–102 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.S. Hubareva, L.S. Svetlichny, 2016, published in Okeanologiya, 2016, Vol. 56, No. 2, pp. 258–265.
Rights and permissions
About this article
Cite this article
Hubareva, E.S., Svetlichny, L.S. Copepods Oithona similis and Oithona davisae: Two strategies of ecological–physiological adaptation in the Black Sea. Oceanology 56, 241–247 (2016). https://doi.org/10.1134/S0001437016020089
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001437016020089