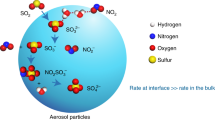
Abstract
Data from field experiments on the dynamics of SO2 oxidation in cloud droplets are presented. The rapid experimentally observed oxidation of SO2 by molecular oxygen is attributed here to the catalytic action of a pair of manganese and iron ions in droplets. Their effect, inhomogeneous in the drop-size distribution and attributed in experiments only to the leaching of ions of these metals from coarse particles of mineral aerosol, is also caused by the transition of the oxidation reaction into the branching mode. The results indicate that the branched regime of catalytic oxidation of SO2 detected in cloud droplets should be considered a new and significant source of sulfates in the atmosphere. This process must be taken into account when considering both the budget of sulfates in the global atmosphere and their impact on the climate.


Similar content being viewed by others
REFERENCES
Alexander, B., Park, R.J., Jacob, D.J., et al., Transition metal-catalyzed oxidation of atmospheric sulfur: global implications for the sulfur budget, J. Geophys. Res.: Atmos., 2009, vol. 114, p. D02309.
Andreae, M.O., Jones, C.D., and Cox, P.M., Strong present-day cooling implies a hot future, Nature, 2005, vol. 435, no. 7046, pp. 1187–1190.
Angle, K.J., Neal, E.E., and Grassian, V.H., Enhanced rates of transition-metal-ion-catalyzed oxidation of S(IV) in aqueous aerosols: Insights into sulfate aerosol formation in the atmosphere, Environ. Sci. Technol., 2021, vol. 55, no. 15, pp. 10291–10299.
Barrie, L.A. and Georgii, H.W., An experimental investigation of the absorption of sulphur dioxide by water drops containing heavy metal ions, Atmos. Environ., 1976, vol. 10, no. 9, pp. 743–749.
Behra, P. and Sigg, L., Evidence for redox cycling of iron in atmospheric water droplets, Nature, 1990, vol. 344, no. 6265, pp. 419–421.
Berdnikov, V.M. and Bazhin, N.M., Redox potentials of some inorganic radicals in aqueous solutions, Zh. Fiz. Khim., 1970, vol. 44, pp. 712–716.
Berglen, T., Berntsen, T., Isaksen, I., and Sundet, J., A global model of the coupled sulfur/oxidant chemistry in the troposphere: The sulfur cycle, J. Geophys. Res., 2004, vol. 109, no. 19, p. D19310.
Berglund, J., Fronaeus, S., and Elding, L.I., Kinetics and mechanism for manganese-catalyzed oxidation of sulfur (IV) by oxygen in aqueous solution, Inorg. Chem., 1993, vol. 32, no. 21, pp. 4527–4537.
Betterton, E.A. and Hoffman, M.R., Oxidation of aqueous SO2 by peroxomonosulfate, J. Phys. Chem., 1988, vol. 92, no. 21, pp. 5962–5965.
Brandt, Ch. and Elding, L.I., Role of chromium and vanadium in the atmospheric oxidation of sulfur(IV), Atmos. Environ., 1998, vol. 32, no. 4, pp. 797–800.
Cheng, Y.F., Zheng, G., Way, Ch., and Mu, Q., Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China, Sci. Adv., 2016, vol. 2, no. 12, p. e1601530.
Coughanowr, D.R. and Krause, F.E., The reaction of SO2 and O2 in aqueous solutions of MnSO4, Ind. Eng. Chem. Fund, 1965, vol. 4, no. 1, pp. 61–66.
Eremina, I.D., Aloyan, A.E., Arutyunyan, V.O., Larin, I.K., Chubarova, N.E., and Ermakov, A.N., Hydrocarbonates in atmospheric precipitation of Moscow: Monitoring data and analysis, Izv., Atmos. Ocean. Phys., 2017, vol. 53, no. 3, pp. 334–342.
Feichter, J., Kjellstrom, E., Rodhe, H., et al., Simulation of the tropospheric sulfur cycle in a global climate model, Atmos. Environ., 1996, vol. 30, nos. 10–11, pp. 1693–1707.
Fomba, K.W., Muller, K., van Pinxteren, D., and Herrmann, H., Aerosol size-resolved trace metal composition in remote northern tropical Atlantic marine environment: Case study Cape Verde Islands, Atmos. Chem. Phys. Discuss., 2013, vol. 13, no. 9, pp. 4801–4814.
Grell, G.A., Peckham, S., Schmitz, R., et al., Fully coupled “online” chemistry within the WRF model, Atmos. Environ., 2005, vol. 39, no. 37, pp. 6957–6975.
Grgić, I., Hudnik, V., Bizjak, M., and Levec, J., Aqueous S(IV) oxidation. I. Catalytic effects of some metal ions, Atmos. Environ., 1991, vol. 25A, no. 8, pp. 1591–1597.
Gröner, E. and Hoppe, P., Automated ion imaging with the nanoSIMS ion microprobe, Appl. Surf. Sci., 2006, vol. 252, no. 19, pp. 7148–7151.
Harris, E., Sinha, B., Hoppe, P., et al., Sulfur isotope fractionation during oxidation of sulfur dioxide: Gas-phase oxidation by oh radicals and aqueous oxidation by H2O2, O3 and iron catalysis, Atmos. Chem. Phys., 2012a, vol. 12, no. 1, pp. 407–423.
Harris, E., Sinha, B., Foley, S., et al., Sulfur isotope fractionation during heterogeneous oxidation of SO2 on mineral dust, Atmos. Chem. Phys., 2012b, vol. 12, pp. 4867–4884.
Harris, E., Sinha, B., van Pinxteren, D., et al., Enhanced role of transition metal ion catalysis during in-cloud oxidation of SO2, Science, 2013, vol. 340, no. 6133, pp. 727–730.
Herrmann, H., Ervens, B., Jacobi, H.-W., et al., Capram2.3: A chemical aqueous phase radical mechanism for tropospheric chemistry, J. Atmos. Chem., 2000, vol. 36, pp. 231–284.
Hung, H.-M., Hsu, M.-N., and Hoffmann, M.R., Quantification of SO2 oxidation on interfacial surfaces of acidic micro-droplets: Implication for ambient sulfate formation, Environ. Sci. Technol., 2018, vol. 52, no. 16, pp. 9079–9086.
Ibusuki, T. and Takeuchi, K., Sulfur dioxide oxidation by oxygen catalyzed by mixtures of manganese(II) and iron(III) in aqueous solutions at environmental reaction conditions, Atmos. Environ., 1987, vol. 21, no. 7, pp. 1555–1560.
Kaplan, D., Himmelblau, D.M., and Kanaoka, C., Oxidation of sulfur dioxide in aqueous ammonium sulfate aerosols containing manganese as a catalyst, Atmos. Environ., 1981, vol. 15, no. 5, pp. 763–773.
Kulmala, M., Pirjola, U., and Mäkelä, U., Stable sulphate clusters as a source of new atmospheric particles, Nature, 2000, vol. 404, no. 6773, pp. 66–69.
Laj, P., Fuzzi, S., Facchini, M.C., et al., Cloud processing of soluble gases, Atmos. Environ., 1997, vol. 31, no. 16, pp. 2589–2598.
Lee, J.K., Samanta, D., Nam, H.G., and Zare, R.N., Micrometer-sized water droplets induce spontaneous reduction, J. Am. Chem. Soc., 2019, vol. 141, no. 27, pp. 10585–10589.
Liu, P., Ye, C., Xue, Ch, Zhang, Ch., Mu, Yu., and Sun, X., Formation mechanisms of atmospheric nitrate and sulfate during the winter haze pollution periods in Beijing: Gas-phase, heterogeneous and aqueous-phase chemistry, Atmos. Chem. Phys., 2020, vol. 20, no. 7, pp. 4153–4165.
Liu, T., Clegg, S.L., and Abbatt, J.P.D., Fast oxidation of sulfur dioxide by hydrogen peroxide in deliquesced aerosol particles, Proc. Natl. Acad. Sci. U. S. A., 2020, vol. 117, no. 3, pp. 1354–1359.
Martin, L.R. and Good, T.W., Catalyzed oxidation of sulfur dioxide in solution: the iron-manganese synergism, Atmos. Environ., 1991, vol. 25A, no. 10, pp. 2395–2399.
Mauldin, R.L., Madronich, S., Flocke, S.J., et al., New insights on OH: Measurements around and in clouds, Geophys. Res. Lett., 1997, vol. 24, no. 23, pp. 3033–3036.
McCabe, J.R., Savarino, J., Alexander, B., et al., Isotopic constraints on non-photochemical sulfate production in the arctic winter, Geophys. Res. Lett., 2006, vol. 33, no. 5, p. L05810.
Pozzoli, L., Bey, I., Rast, S., Schultz, M.G., Stier, P., and Feichter, J., Trace gas and aerosol interactions in the fully coupled model of aerosol–chemistry–climate ECHAM5-HAMMOZ: 1. Model description and insights from the spring 2001 TRACE-P experiment, J. Geophys. Res., 2008, vol. 113, p. D07308.
Sedlak, D.L., Hoigne, J., David, M.M., et al., The cloudwater chemistry of iron and copper at Great Dun Fell, U.K., Atmos. Environ., 1997, vol. 31, no. 16, pp. 2515–2526.
Seinfeld, J.H. and Pandis, S.N., Atmospheric Chemistry and Physics, from Air Pollution to Climate Change, Hoboken, N.J.: John Wiley and Sons, 2016.
Tilgner, A., Bräuer, P., Wolke, R., and Herrmann, H., Modelling multiphase chemistry in deliquescent aerosols and clouds using CAPRAM3.0i, J. Atmos. Chem., 2013, vol. 70, no. 3, pp. 221–256.
van Eldik, R., Coichev, N., Reddy, K.B., and Gerhard, A., Metal ion catalyzed autoxidation of sulfur(IV) oxides: Redox cycling of metal ions induced by sulfite, Ber. Bunsen-Ges. Phys. Chem., 1992, vol. 96, no. 3, pp. 478–481.
Wang, G.H., Zhang, R.Y., Gomes, M.E., et al., Persistent sulfate formation from London fog to Chinese haze, Proc. Natl. Acad. Sci. USA, 2016, vol. 113, no. 48, pp. 13630–13635.
Warneck, P., Mirabel, P., Salmon, G.A., et al., Review of the activities and achievements of the EUROTRAC subproject HALIPP, in Heterogeneous and Liquid Phase Processes, Warneck, P., Ed., Berlin: Springer, 1996, p. 7.
Winterholler, B., Hoppe, P., Foley, S., and Andreae, M.O., Sulfur isotope ratio measurements of individual sulfate particles by NanoSIMS, Int. J. Mass Spectrom., 2008, vol. 272, no. 1, pp. 63–77.
Xie, Y.Z., Liu, Z.R., Wen, T.X., et al., Characteristics of chemical composition and seasonal variations of PM2.5 in Shijiazhuang, China: Impact of primary emissions and secondary formation, Sci. Total Environ., 2019, vol. 677, pp. 215–229.
Yermakov, A.N., On the influence of ionic strength on the kinetics of sulfite oxidation in the presence of Mn(II), Kinet. Catal., 2022, vol. 63, no. 2, pp. 157–165.
Yermakov, A.N. and Purmal, A.P., Catalysis of oxidation HSO3 -/SO3 2- by manganese ions, Kinet. Catal., 2002, vol. 43, no. 2, pp. 249–260.
Yermakov, A.N., Aloyan, A.E., and Arutyunyan, V.O., Dynamics of sulfate origination in atmospheric haze, Opt. Atmos. Okeana, 2023, vol. 36, no. 2, pp. 148–153.
Zhang, H., Xu, Y., and Jia, L., A chamber study of catalytic oxidation of SO2 by Mn2+/Fe3+ in aerosol water, Atmos. Environ., 2021, vol. 245, p. 118019.
Zheng, G.J., Duan, F.K., Su, H., et al., Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions, Atmos. Chem. Phys., 2015, vol. 15, no. 6, pp. 2969–2983.
Funding
This work was performed with funds from a state assignment for the Talrose Institute for Energy Problems of Chemical Physics, Russian Academy of Sciences, theme 122040400095-79.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Nikol’skii
Rights and permissions
About this article
Cite this article
Yermakov, A.N., Aloyan, A.E., Arutyunyan, V.O. et al. On the Mechanism of Sulfur Dioxide Oxidation in Cloud Drops. Izv. Atmos. Ocean. Phys. 59, 538–547 (2023). https://doi.org/10.1134/S0001433823050055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001433823050055