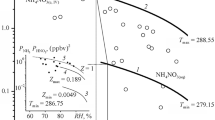
Abstract
Monitoring data on the ion composition of aerosols and gas admixtures in the background and polluted atmosphere of the Lake Baikal region are presented. The ion composition and morphology of aerosols are affected by heterogeneous chemical reactions and variations in relative humidity. Two types of aerosol particles are revealed over this region. The fraction of solid particles recorded in most episodes includes primarily carbonates of alkaline and alkaline-earth metals. With increased atmospheric humidity, these particles are engaged in heterogeneous chemical reactions with gas-phase NH3 and H2SO4, proceeding through the phase of watering. As a result, the composition of these aerosols is changed, and a fraction of aqueous H2O/H2SO4/(NH4)2SO4 aerosol particles of a different composition is formed. On the basis of a physical and chemical analysis of monitoring data on the aerosol composition and concentrations of gas admixtures, the average aerosol-size distribution of different types is estimated. For the first time, the mean acidity of aqueous aerosol particles is estimated.
Similar content being viewed by others
References
“Joint EUROTRAC/CEC Projects, General Report,” in Annual Report 1992 (Garmish-Partenkirchen, 1993), Part 6, pp. 1–17.
Atmos. Chem. Phys. Discuss., Special Issue, No. 2 (2002) (http://www.atmos-chem-phys.acpd/2/).
K. Koutsenogii, P. Koutsenogii, B. Smolyakov, and T. Khodgher, “Monitoring of Spatial and Temporal Variation of the Particle Size Distribution and Chemical Composition of the Atmospheric Aerosol in Siberia,” in Global Atmospheric Change and Its Impact on Regional Air Quality, NATO Science Series, Series IV: Earth and Environmental Sciences, Ed. by I. Barnes (Kluwer, Dordrecht, 2002), Vol. 16, pp. 229–235.
“Atmospheric Aerosols in Siberia,” Certificate of RosAPO RF Database No. 990012, Possessor of Right Limnological Institute, Russian Academy of Sciences, Siberian Division (1999), Appl. No. 990001, Russia (10 March, 1999).
T. V. Khodzher, L. P. Golobokova, V. A. Obolkin, et al., “Diurnal and Seasonal Variability of the Ion Composition of Atmospheric Aerosols in Southeastern Siberia,” Opt. Atmos. Okeana 10, 650–655 (1997).
Manual for Sampling and Chemical Analysis, EMEP/CCC Report no. 1/95/0-7726 (1995).
G. S. Fomin, Water: Control of Chemical, Bacterial, and Radiation Safety According to International Standards (Moscow, 2000) [in Russian].
G. I. Baram, A. L. Vereshchagin, and L. P. Golobokova, “Microcolumn High-Performance Liquid Chromatography with UV Detection for the Determination of Anions in Environmental Materials,” J. Anal. Chem. 5, 854–857 (1999).
A. L. Vereshchagin, V. F. Dudinskii, L. P. Golobokova, et al., “Determination of UV-Absorbing Anions in Environmental Samples by Microcolumn High-Performance Liquid Chromatography,” J. Anal. Chem. 55, 1000–1002 (2000).
Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling (Jet Propulsion Laboratory Publication, 1997), No. 12.
C. A. Cantrell, R. E. Shetter, T. M. Gilpin, and J. G. Calvert, “Peroxy Radicals Measured During Mauna Loa Observatory Photochemistry Experiment, 2: The Data and First Analysis,” J. Geophys. Res. D 101, 14643–14652 (1996).
S. L. Clegg and P. Brimblecumbe, “Solubility of Volatile Electrolytes in Multicomponent Solutions with Atmospheric Applications,” ACS Symp. Ser. 416, 58–73 (1990).
H. Herrmann, B. Ervens, H.-W. Jacobi, et al., “CAPRAM2.3: A Chemical Aqueous Phase Radical Mechanism for Troposheric Chemistry,” J. Atmos. Chem. 36, 231–284 (2000).
S. E. Schwartz, Acid Precipitation Series, Ed. by J. G. Calvert (Butterworth, Boston, 1984).
S. L. Clegg, P. Brimblecombe, and A. S. Wexler, “A Thermodynamic Model of the System H+-NH +4 -SO 2−4 -NO −3 -H2O at Tropospheric Temperatures,” J. Phys. Chem. A 102, 2137–2154 (1998).
A. S. Wexler and S. L. Clegg, “Atmospheric Aerosol Models for System Including Ions H+, NH +4 , Na+, SO 2−4 , NO −3 , Cl−, Br− and H2O,” J. Geophys. Res. D 107 (2002).
Handbook for Chemists (Gos. Nauchn.-Tekh. Izd. Khim. Literatury, Leningrad, 1952), Vol. 3 [in Russian].
D. R. Hanson and E. Kosciuch, “The NH3 Mass Accommodation Coefficient for Uptake onto Sulfuric Acid Solutions,” J. Phys. Chem. A 107, 2199–2208 (2003).
G. P. Brasseur, J. J. Orlando, and G. S. Tyndall, Atmospheric Chemistry and Global Change (Oxford Univ. Press, New York, 1999).
P. Laj, S. Fuzzi, M. C. Facchini, et al., “Experimental Evidence for In-Cloud Production of Aerosol Sulphate,” Atmos. Environ. 31, 2503–2514 (1997).
P. K. Kutsenogii, N. S. Bufetov, V. I. Drosdova, et al., “Ion Composition of Atmospheric Aerosol near Lake Baikal,” Atmos. Environ., 27 (1993).
R. D. Latterman, Calcium Dissolution Rate in Limestone Contactors (Syracuse Univ., Syracuse, New York, 1995), pp. 1–112.
M. Liler, Reaction Mechanisms in Sulphuric Acid (Academic, London, 1971).
K. S. Pitzer, R. N. Roy, and L. F. Silvestor, “Thermodynamics of Electrolytes. 7. Sulfuric Acid,” J. Am. Chem. Soc. 99, 4930–4936 (1977).
D. R. Hanson, “Mass Accommodation of H2SO4 and CH3SO3H on Water-Sulfuric Acid Solutions from 6 to 97% RH,” J. Phys. Chem. A 109, 6919–6927 (2005).
V. I. Alekseev, Quantitative Analys (Goskhimizdat, Moscow, 1954) [in Russian].
N. A. Kendal and S. T. Martin, “Mobile Ions on Carbonic Surfaces,” Geochim. Cosmochim. Acta 69, 3257–3263 (2005).
M. Wallin and I. Bjerle, “Rate Model for Limestone Dissolution: A Comparison,” Geochem. Cosmochim. Acta 53, 1171–1176 (1989).
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.N. Yermakov, A.E. Aloyan, T.V. Khodzer, L.P. Golobokova, V.O. Arutyunyan, 2007, published in Izvestiya AN. Fizika Atmosfery i Okeana, 2007, Vol. 43, No. 2, pp. 234–245.
Rights and permissions
About this article
Cite this article
Yermakov, A.N., Aloyan, A.E., Khodzer, T.V. et al. On the influence of atmospheric chemical reactions on the ion composition of aerosol particles in the Baikal region. Izv. Atmos. Ocean. Phys. 43, 208–218 (2007). https://doi.org/10.1134/S0001433807020077
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S0001433807020077