Abstract
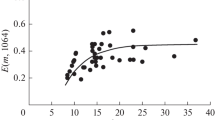
The optical coefficients of soot particles are measured in a flow-through optical cell at the wavelength 635 nm in a dry atmosphere and in an atmosphere saturated with water vapor. Two types of systems modeling atmospheric soot aerosols and differing in the degree of hygroscopicity of their particles are investigated and compared. One of the systems contains hydrophobic acetylene soot, and the other contains hydrophilic soot obtained through modification of the initial soot by vapors of glutaric acid. The results show that the optical properties of hydrophobic soot depend only slightly on the conditions of moistening, whereas the optical properties of hydrophilic soot change abruptly upon its moistening because of the formation of a hydrate shell. In the atmosphere saturated with water-vapor, the monomolecular layer of a hydrophilic organic substance leads to the watering of particles and an abrupt (more than twofold) increase in the cross section of light scattering. A further growth of the hydrophilic component of soot particles initiates the formation of micron drops on them, thus resulting not only in the natural effect of light scattering enhancement but also in a noticeable light absorption increase. In particular, a light absorption enhancement by a factor of 3.5 is characteristic of particles of enriched hydrophilic soot.
Similar content being viewed by others
References
T. A. Tarasova, I. A. Gorchakova, M. A. Sviridenkov, et al., “Estimation of the Radiative Forcing of Smoke Aerosol from Radiation Measurements at the Zvenigorod Scientific Station in the Summer of 2002,” Izv. Akad. Nauk, Fiz. Atmos. Okeana 40, 514–524 (2004) [Izv. Atmos. Ocean. Phys. 40, 454–463 (2004)].
V. Ramanathan, P. J. Crutzen, J. Lelieveld, et al., “The Indian Ocean Experiment: An Integrated Analysis of the Climate Forcing and Effects of the Great Indo-Asian Haze,” J. Geophys. Res. D 106, 398 (2001).
R. W. Bergstrom, P. B. Russell, and P. Hignett, “Wavelength Dependence of the Adsorption of Black Carbon Particles: Predictions and Results from TARFOX Experiment and Implications for the Aerosol Single Scattering Albedo,” J. Atmos. Sci. 59, 567–577 (2002).
K. Ya. Kondrat’ev, N. I. Moskalenko, S. V. Skvortsova, et al., “Modeling Optical Characteristics of Soot Aerosol,” Dokl. Akad. Nauk SSSR, Ser. Geofizika. 296, 314–317 (1987).
J. Hansen, M. Sato, and R. Ruedy, “Radiative Forcing and Climate Response,” J. Geophys. Res. D 102, 6831–6864 (1997).
J. M. Haywood, D. L. Roberts, and A. Slingo, “General Circulation Model Calculations of the Direct Radiative Forcing by Anthropogenic Sulfate and Fossil-Fuel Soot Aerosol,” J. Clim. 10, 1562–1577 (1997).
Intergovernmental Panel on Climate Change (IPCC), Climate Change 2001—Scientific Basis, Technical Summary of the Working Group I Report (Cambridge Univ. Press, Cambridge, 2001).
M. Z. Jacobson, “Global Direct Radiative Forcing Due to Multicomponent Anthropogenic and Natural Aerosols,” J. Geophys. Res. D 106, 1551–1568 (2001).
S. H. Chung and J. H. Seinfeld, “Global Distribution and Climate Forcing of Carbonaceous Aerosols,” J. Geophys. Res. D 107, doi: 10.1029/2001JD001397 (2002).
C. Wang, “A Modeling Study on the Climate Impacts of Black Carbon Aerosols,” J. Geophys. Res. 109, D03106, doi: 10.1029/2003JD004084 (2004).
S. L. Clegg and K. S. Pitzer, “Thermodynamics of Multicomponent, Miscible, Ionic Solutions: Generalised Equations for Symmetrical Electrolytes,” J. Phys. Chem. 96, 3513–3520 (1992).
S. L. Clegg, P. Brimblecombe, and A. S. Wexler, “Thermodynamic Model of the System H+-NH +4 -SO 2−4 -NO3-H2O at Tropospheric Temperatures,” J. Phys. Chem. A 102, 2137–2154 (1998).
E. F. Mikhailov and S. S. Vlasenko, “Structure and Optical Properties of Soot Aerosol in a Moist Atmosphere: Part 1. Change in the Structure of Soot Particles in the Process of Condensation,” Izv. Akad. Nauk, Fiz. Atmos. Okeana 43 (2007) [Izv., Atmos. Ocean. Phys. 43, 195–207 (2007).
C. L. Erlick, L. M. Russell, and A. Ramaswamy, “A Microphysics-Based Investigation of the Radiative Effects of Aerosol-Cloud Interactions,” J. Geophys. Res. A 106, 1249–1270 (2001).
M. L. Pitchford and P. H. McMurry, “Relationship Between Measured Water Vapor Growth and Chemistry of Atmospheric Aerosol for Grand Canyon, Arizona, in Winter 1990,” Atmos. Environ. 28, 827–839 (1994).
M. Gysel, S. Nyeki, E. Weingarthner, et al., “Properties of Jet Engine Combustion Particles during the PartEmis Experiment: Hygroscopicity at Subsaturated Conditions,” Geophys. Res. Lett. 30, doi: 10.1029/2003GL016896 (2003).
E. F. Mikhailov, S. S. Vlasenko, A. A. Kiselev, and T. I. Ryshkevich, “Change in the Structure of Fractal Soot Particles under the Effect of Capillary Forces: Experimental Results,” Kolloidn. Zh. 59, 195–203 (1997).
C. G. Peng, M. N. Chan, and C. K. Chan, “The Hygroscopic Properties of Dicarboxylic and Multifunctional Acids: Measurements and UNIFAC Predictions,” Environ. Sci. Technol. 35, 4495–4501 (2001).
I. T. Goronovskii, Yu. P. Nazarenko, and E. F. Nekryach, Short Handbook of Chemistry (Naukova Dumka, Kiev, 1974) [in Russian].
R. A. Dobbings, G. W. Mulholland, and N. P. Bryner, “Comparison of a Fractal Smoke Optics Model with Light Extinction Measurements,” Atmos. Environ. 28, 889–897 (1994).
J. Zhu, M. Y. Choi, G. W. Milholland, and L. A. Gritzo, “Soot Scattering Measurements in the Visible and Near-Infrared Spectrum,” in Proceedings of 28th International Symposium on Combustion (2002), pp. 439–446.
J. Nelson, “Test of a Mean Field Theory for the Optics of Fractal Clusters,” J. Modern Opt. 36, 1031–1057 (1989).
H. Y. Chen, M. F. Iskander, and J. E. Penner, “Light Scattering and Absorption by Fractal Agglomerates and Coagulations of Smoke Aerosols,” J. Modern Opt. 37, 171–181 (1990).
U. O. Koylu and G. M. Faeth, “Optical Properties of Overfire Soot in Buoyant Turbulent Diffusion Flames at Long Residence Times,” J. Heat Transfer 116, 152–159 (1994).
M. H. Schnaiter, H. Horvath, O. Mohler, et al., “UV-VIS-NIR Spectral Optical Properties of Soot and Soot-Containing Aerosols,” J. Aeros. Sci 34, 1421–1444 (2003).
C. E. Corrigan and T. Novakov, “Cloud Condensation Nucleus Activity of Organic Compounds: A Laboratory Study,” Atmos. Environ. 33, 2661–2668 (1999).
C. N. Cruz and S. N. Pandis, “A Study of the Ability of Pure Secondary Organic Aerosol to Act As Cloud Condensation Nuclei,” Atmos. Environ. 31, 2205–2214 (1997).
P. P. Kumar, K. Broekhuizen, and J. P. D. Abbatt, “Organic Acids As Cloud Condensation Nuclei: Laboratory Studies of Highly Soluble and Insoluble Species,” Atmos. Chem. Phys. 3, 509–520 (2003).
K. A. Fuller, “Effects of Mixing on Extinction by Carbonaceous Particles,” J. Geophys. Res. D 109, 15941–15954 (1999).
V. M. Markel, “The Effects of Averaging on the Enhancement Factor for Absorption of Light by Carbon Particles in Microdroplets of Water,” J. Quantum Spectroscopy Radiat. Transfer 72, 765–774 (2002).
K. S. Pitzer, “Thermodynamics of Electrolytoes. I. Theoretical Basis and General Equations,” J. Chem. Phys. 77, 268–277 (1973).
I. Mokbel, S. Ye, J. Jose, and P. Xans, “Study of Non Ideality of Various Aqueous Sodium Chlorid Solutions by Vapor Pressures Measurements and Correlation of Experimental Results by Pitzer’s Method,” J. Chim. Phys. 94, 122–137 (1997).
M. Z. Jacobson, “A Physically-Based Treatment of Elemental Carbon Optics: Implications for Global Direct Forcing of Aerosols,” Geophys. Res. Let. 27, 217–220 (2000).
P. T. Ackerman and O. B. Toon, “Absorption of Visible Radiation in Atmosphere Containing Mixtures of Absorbing and Nonabsorbing Particles,” Appl. Opt. 20, 3661–3668 (1981).
Author information
Authors and Affiliations
Additional information
Original Russian Text © E.F. Mikhailov, S.S. Vlasenko, 2007, published in Izvestiya AN. Fizika Atmosfery i Okeana, 2007, Vol. 43, No. 2, pp. 221–233.
Rights and permissions
About this article
Cite this article
Mikhailov, E.F., Vlasenko, S.S. Structure and optical properties of soot aerosol in a moist atmosphere: 2. Influence of hydrophilicity of particles on the extinction, scattering, and absorption coefficients. Izv. Atmos. Ocean. Phys. 43, 195–207 (2007). https://doi.org/10.1134/S0001433807020065
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S0001433807020065