Abstract
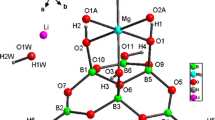
Crystals of new borate KTm[B4O6(OH)4] ⋅ 3H2O (sp. gr. Р\(\bar {3}\)1m, a = 4.5472(7) Å, c = 12.151(3) Å) have been obtained under hydrothermal conditions at T = 280°C and P = 100 atm. Their structure contains packets of two polar mica-like tetrahedral layers [B4O6(OH)4]∞∞, connected by TmO6 octahedra. The interpacket space is occupied statistically by K atoms and water molecules. A similar layer is typical of peprossiite and its synthetic analogue NdAl2.07[B4O10]O0.6 and KTa[B4O6(OH)4](OH)2 ⋅ 1.33H2O, which have another sp. gr. P\(\bar {6}\)2m and trigonal-prismatic coordination of heavy atoms. The structure of the new compound is compared with two other structures of this family: KGd[B6O10(OH)2] and KHo[B6O10(OH)2]. A previously unknown combination of simple mica-like layers and octahedra in one packet is implemented in the new member of the family. All compounds under consideration are characterized by disorder and statistical occupancy of sites, especially in the interpacket space. The KTm[B4O6(OH)4] ⋅ 3H2O crystals demonstrate strong emission of blue light due to the radiative 4f–4f transitions of the Tm3+ cation. The most intense transition in the photoluminescence spectrum is 1D2–3F4 at 450 nm.





Similar content being viewed by others
REFERENCES
The Cambridge Crystallographic Data Centre (CCDC). Inorganic Crystal Structure Data Base (ICSD). http://www.ccdc.cam.ac.uk/; http://www.fiz-karlsruhe.de.
Crystallography Open Database. http://www.crystallography.net/cod.
C. L. Christ and J. R. Clark, Phys. Chem. Miner. 2, 59 (1977).
R. E. Newnham, M. J. Redman, and R. P. Santoro, J. Am. Ceram. Soc. 46, 253 (1963).
Y. Liu, F. Yu, Zh. Wang, et al., Cryst. Eng. Commun. 16, 7141 (2014).
A. B. Ilyukhin and B. F. Dzhurinskii, Zh. Neorg. Khim. 38, 1625 (1993).
E. L. Belokoneva, A. P. Zorina, and O. V. Dimitrova, Crystallogr. Rep. 58 (2), 210 (2013).
M. H. Moeller, T. Schleid, H. Emme, et al., Z. Natur. B 59, 202 (2004).
H. Emme, M. Valldor, R. Pöttgen, and H. Huppertz, Chem. Mater. 17, 2707 (2005).
F. Liebau, Structural Chemistry of Silicates: Structure, Bonding and Classification (Springer, New York, 1985; Mir, Moscow, 1988).
X. Qiao, Y. Cheng, L. Qin, et al., J. Alloys Compd. 617, 946 (2015).
P. Du and J. S. Yu, Mater. Res. Bull. 84, 303 (2016).
I. V. Nikiforov, D. V. Deyneko, D. A. Spassky, et al., Mater. Res. Bull. 130, 110925 (2020).
Z. Xue, Z. Yi, X. Li, et al., Biomaterials 115, 90 (2017).
H. Zhang, Y. Li, Y. Lin, et al., Nanoscale 3, 963 (2011).
W. T. Carnall, P. R. Fields, and K. Rajak, J. Chem. Phys. 49, 4424 (1968). https://doi.org/10.1063/1.1669893
A. Tymiński and T. Grzyb, J. Lumin. 181, 411 (2017).
J. B. Gruber and J. G. Conway, J. Chem. Phys. 32 (4), 1178 (1960).
A. Nadort, J. Zhao, and E. M. Goldys, Nanoscale 8, 13099 (2016).
Transition Metal and Rare Earth Compounds: Excited States, Transitions, Interactions, Vol. I, Ed. by H. Yersin (Springer, New York, 2001).
M. Runowski, A. Shyichuk, A. Tymiński, et al., ACS Appl. Mater. Interfaces 10 (20), 17269 (2018).
S. Yu. Stefanovich, Extended Abstracts of Eur. Conf. on Lasers and ElecrtoOptics (CLEO Europe’94), Amsterdam, 1994, p. 249.
E. L. Belokoneva, S. Yu. Stefanovich, and O. V. Dimitrova, J. Solid State Chem. 195, 79 (2002).
E. L. Belokoneva, A. P. Topnikova, S. Yu. Stefanovich, et al., Solid State Sci. 46, 43 (2015).
Agilent, CrysAlis PRO (Agilent Technologies Ltd, Yarnton, Oxfordshire, England, 2014).
G. M. Sheldrick, Acta Crystallogr. A 64, 112 (2008).
L. J. Farrugia, J. Appl. Crystallogr. 45, 849 (2012).
G. M. Sheldrick, Acta Crystallogr. C 71, 3 (2015).
E. Dowty, ATOMS. Shape Software (Kingsport, Tennessee, USA, 2006).
G. Dominiak-Dzik, W. Ryba-Romanowski, S. Goł, and A. Pajaczkowska, J. Phys.: Condens. Matter 12, 5495 (2000).
A. N. Meza-Rocha, A. Speghini, R. Lozada-Morales, and U. Caldiño, Opt. Mater. 58, 183 (2016).
M. Que, Zh. Ci, Yu. Wang, et al., J. Lumin. 144, 64 (2013).
D. Yu. Pushcharovskii, O. G. Karpov, N. I. Leonyuk, and N. V. Belov, Dokl. Akad. Nauk SSSR, 241, 91 (1978).
ACKNOWLEDGMENTS
We are grateful to S.Yu. Stefanovich for the SHG measurements and consultations and to V.O. Yapaskurt for the determination of the crystal composition.
Funding
This study was performed in part within a state contract for the Institute of Solid State Physics of the Russian Academy of Sciences. The analysis of the luminescence properties was supported by the Russian Science Foundation (grant no. 19-77-10013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Sin’kov
Rights and permissions
About this article
Cite this article
Topnikova, A.P., Belokoneva, E.L., Dimitrova, O.V. et al. KTm[B4O6(OH)4] ⋅ 3H2O: A New Member of Borate Family with Mica-like Tetrahedral Layers. Crystallogr. Rep. 66, 105–111 (2021). https://doi.org/10.1134/S1063774521010193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774521010193