Abstract
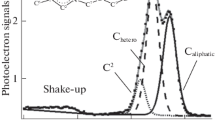
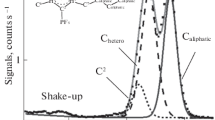
In this study, six 1-butyl-2,3-dimethylimidazolium ionic liquids are analyzed by X-ray photoelectron spectroscopy. A reliable fitting model is developed for the C 1s region. The charge correction method is discussed in detail. It suggests that the aliphatic carbon component can be used as an internal reference only if the Kamlet-Taft hydrogen bond acceptor ability value is larger than 0.57. The electronic environment of each anion-based component is compared to that of 1-butyl-3-methylimidazolium ionic liquids. It is found that noticeable binding energy shift can be observed in the spectra of S 2p for [TfO]– and P 2p for [PF6]–.





Similar content being viewed by others
REFERENCES
E. F. Smith, I. J. Villar-Garcia, D. Briggs, and P. Licence, Chem. Commun., No. 45, 5633 (2005).
S. Caporali, U. Bardi, and A. Lavacchi, J. Electron Spectrosc. Relat. Phenom. 151, 4 (2006).
T. Cremer, C. Kolbeck, K. R. J. Lovelock, N. Paape, R. Wölfel, P. S. Schulz, P. Wasserscheid, H. Weber, J. Thar, B. Kirchner, F. Maier, and H.-P. Steinrück, Chem.-Eur. J. 16, 9018 (2010).
I. J. Villar-Garcia, E. F. Smith, A. W. Taylor, F. Qiu, K. R. J. Lovelock, R. G. Jones, and P. Licence, Phys. Chem. Chem. Phys. 13, 2797 (2011).
D. Briggs and J. T. Grant, Surface Analysis by Auger and X-ray Photoelectron Spectroscopy (IM Publ., Manchester, 2003).
R. T. Lewis and M. A. Kelly, J. Electron Spectrosc. Relat. Phenom. 20, 105 (1980).
J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker, and R. D. Rogers, Green Chem. 3, 156 (2001).
A. W. Taylor, K. R. J. Lovelock, A. Deyko, P. Licence, and R. G. Jones, Phys. Chem. Chem. Phys. 12, 1772 (2010).
C. D. Wagner, L. E. Davis, M. V. Zeller, J. A. Taylor, R. H. Raymond, and L. H. Gale, Surf. Interface Anal. 3, 211 (1981).
G. Beamson, D. T. Clark, J. Kendrick, and D. Briggs, J. Electron Spectrosc. Rel. Phenom. 57, 79 (1991).
D. Briggs and G. Beamson, Anal. Chem. 64, 1729 (1992).
S. Spange, R. Lungwitz, and A. Schade, J. Mol. Liq. 192, 137 (2014).
A. W. Taylor, F. L. Qiu, I. J. Villar-Garcia, and P. Licence, Chem. Commun., No. 39, 5817 (2009).
S. Men, K. R. J. Lovelock, and P. Licence, Phys. Chem. Chem. Phys. 13, 15244 (2011).
I. J. Villar-Garcia, K. R. J. Lovelock, S. Men, and P. Licence, Chem. Sci. 5, 2573 (2014).
S. Men, J. Rong, T. Zhang, X. Wang, L. Feng, C. Liu, and Y. Jin, Russ. J. Phys. Chem. A 92, 1975 (2018).
ACKNOWLEDGMENTS
The authors thank Liaoning Provincial Foundation of Science and Technology (20180550482) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li Wei, Shuang Men X-ray Photoelectron Spectroscopy of 1-Butyl-2,3-Dimethylimidazolium Ionic Liquids: Charge Correction Methods and Electronic Environment of the Anion. Russ. J. Phys. Chem. 93, 2676–2680 (2019). https://doi.org/10.1134/S0036024419130326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419130326