Abstract
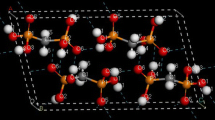
First-principle calculations are performed on the dimers of 2,6-diamino-3,5-dinitropyridine (ANPy) and its N-oxide (2,6-diamino-3,5-dinitropyridine-1-oxide, ANPyO). The dimers as well as the monomers are fully optimized by the DFT-B3LYP and HF methods in conjunction with 6-311G**, 6-311++G**, and cc-pVDZ basis sets. The N-O bond length of the pyridine N-oxide moiety decreases in the ANPyO dimer in the dimerization process, which results in a larger deformation energy of the ANPyO submolecule. This deformation prevents the submolecules from further close contact and the formation of strong H-bonds between the nitro and amino groups. The optimized intermolecular distances of the ANPyO dimer are in good agreement with the corresponding experimental values. There is a weak C-H...O hydrogen bond in the ANPyO dimer; the B3LYP method underestimates its binding energy. On the contrary, for the ANPy dimer, the binding energy obtained at the B3LYP level is larger than that obtained at the HF level. The individual O...H strength is stronger in the ANPy dimer than that in ANPyO, which is consistent with the O...H distance. The O...H-C type of the H-bond is stronger in the ANPyO dimer than the ordinary O...H-C bond due to the N-oxide oxygen atom bearing larger negative charges. The corrected binding energy for each hydrogen bond between nitro oxygen and amino hydrogen is about −5 kJ/mol in the ANPy dimer, which is stronger than that in the ANPyO dimer.
Similar content being viewed by others
References
K. L. Anderson, L. H. Merwin, W. S. Wilson, and J. C. Facelli, Int. J. Mol. Sci., 3, 858–872 (2002).
J. S. Li, Y. G. Huang, and H. S. Dong, Chin. J. Energetic Mater., 12, 576–579 (2004).
Y. H. Wang, Z. Hu, J. Long, X. L. Meng, Y. J. Song, and Y. D. Huang, J. Chem. Eng. Data, 55, 561–565 (2010).
J. Cheng, Q. Z. Yao, X. L. Zhou, Y. Du, D. Fang, and Z. L. Liu, Chin. J. Org. Chem., 28, 1943–1947 (2008).
P. F. Pagoria, G. S. Lee, and A. R. Mitchell, Thermochim. Acta, 384, 187–204 (2002).
Z. W. He, J. Cheng, and Z. L. Liu, Chin. J. Energetic Mater., 17, 392–395 (2009).
X. H. Zhang, L. X. Ding, and G. J. Zh, Preparation Methods of Explosive in the Lab, National Defense Industrial Press, Beijing (1997).
S. F. Bureiko and S. Y. Kucherov, J. Struct. Chem., 50, 712–721 (2009).
A. N. Pankratov, N. A. Bychkov, and O. M. Tsivileva, J. Struct. Chem., 51, 9–15 (2010).
R. W. Shaw, T. B. Brill, and D. L. Thompson, Overviews of Recent Research on Energetic Materials, World Scientific Publishing Company, Singapore (2005).
J. P. Foster and F. Weinhold, J. Am. Chem. Soc., 102, 7211–7218 (1980).
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev., 88, 899–926 (1988).
G. Chalasinski and M. M. Szczesniak, Chem. Rev., 100, 4227–4252 (2000).
X. H. Ju and H. M. Xiao, J. Mol. Struct. (Theochem.), 588, 79–86 (2002).
D. Strack, Phytochemistry, 57, 144 (2001).
H. B. Schlegel, J. Comp. Chem., 3, 214–218 (1982).
A. Johansson, P. Kollman, and S. Rothenberg, Theor. Chem. Acta, 29, 167–172 (1973).
S. F. Boys and F. Bernardi, Mol. Phys., 19, 553–566 (1970).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh PA (2003).
R. A. Hollins, R. A. Nissan, W. S. Wilson, and R. D. Gilardi, 2,6-Diamino-3,5-dinitropyridine-1-oxide: A New Insensitive Explosive, NAWCWPNS Technical Publication: China Lake, CA (1995).
B. Paizs and S. Suhai, J. Comp. Chem., 19, 575–584 (1998).
Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 120, 215–241 (2008).
M. W. Feyereisen, D. Feller, and D. A. Dixon, J. Phys. Chem., 100, 2993–2997 (1996).
R. F. Bader, Atoms in Molecules: A Quantum Theory, Clarendon Press, Oxford (1990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2012 by L.-F. Xie, C.-C. Ye, X.-H. Ju, F.-Q. Zhao
__________
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 53, No. 4, pp. 672–677, July–August, 2012.
Rights and permissions
About this article
Cite this article
Xie, L.F., Ye, C.C., Ju, X.H. et al. Theoretical study on dimers of 2,6-diamino-3,5-dinitropyridine and its N-oxide. J Struct Chem 53, 659–664 (2012). https://doi.org/10.1134/S0022476612040075
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476612040075