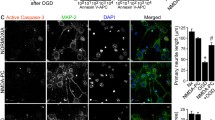
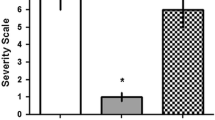
Abstract
Preconditioning (PC) is one of the most effective strategies to reduce the severity of cell damage, in particular of nervous tissue cells. Although PC mechanisms are studied insufficiently, it is clear that proteases are involved in them, but their role has yet been not studied in detail. In this work, some mechanisms of a potential recruiting of proteases in PC are considered. Our attention is mainly focused on the protease families of caspases and cathepsins and on protease receptors. We present evidence that just these proteins are involved in the PC of brain cells. A hypothesis is proposed that secreted cathepsin B is involved in the realization of PC through activation of PAR2 receptor.
Similar content being viewed by others
Abbreviations
- AP:
-
autophagy
- IPC:
-
ischemic preconditioning
- PC:
-
preconditioning
References
Dave, K. R., Saul, I., Prado, R., Busto, R., and Perez-Pinzon, M. A. (2006) Remote organ ischemic preconditioning protects brain from ischemic damage following asphyxial cardiac arrest, Neurosci. Lett., 404, 170–175.
Hahn, C. D., Manlhiot, C., Schmidt, M. R., Nielsen, T. T., and Redington, A. N. (2011) Remote ischemic pre-conditioning: a novel therapy for acute stroke? Stroke, 42, 2960–2962.
Malhotra, S., Naggar, I., Stewart, M., and Rosenbaum, D. M. (2011) Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury, Brain Res., 1386, 184–190.
Hu, S., Dong, H., Zhang, H., Wang, S., Hou, L., and Chen, S. (2012) Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats, Brain Res., 1459, 81–90.
Dirnagl, U., Becker, K., and Meisel, A. (2009) Preconditioning and tolerance against cerebral ischemia: from experimental strategies to clinical use, Lancet Neurol., 8, 398–412.
Koch, S. (2010) Preconditioning the human brain: practical considerations for proving cerebral protection, Transl. Stroke Res., 1, 161–169.
Obrenovitch, T. P. (2008) Molecular physiology of preconditioning-induced brain tolerance to ischemia, Physiol. Rev., 88, 211–247.
Kitagawa, K., Matsumoto, M., Tagaya, M., Hata, R., Ueda, H., Niinobe, M., Handa, N., Fukunaga, R., Kimura, K., Mikoshiba, K., and Kamada, T. (1990) “Ischemic tolerance” phenomenon found in the brain, Brain Res., 528, 21–24.
Stagliano, N. E., Perez-Pinzon, M. A., Moskowitz, M. A., and Huang, P. L. (1999) Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice, J. Cereb. Blood Flow Metab., 19, 757–761.
Bruer, U., Weih, M. K., Isaev, N. K., Meisel, A., Ruscher, K., Bergk, A., Trendelenburg, G., Wiegand, F., Victorov, I. V., and Dirnagl, U. (1997) Induction of tolerance in rat cortical neurons: hypoxic preconditioning, FEBS Lett., 414, 117–121.
Grabb, M. C., and Choi, D. W. (1999) Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors, J. Neurosci., 19, 1657–1662.
Sakaki, T., Yamada, K., Otsuki, H., Yuguchi, T., Kohmura, E., and Hayakawa, T. (1995) Brief exposure to hypoxia induces bFGF mRNA and protein and protects rat cortical neurons from prolonged hypoxic stress, Neurosci. Res., 23, 289–296.
Romera, C., Hurtado, O., Botella, S. H., Lizasoain, I., Cardenas, A., and Fernandez-Tome, P. (2004) In vitro ischemic tolerance involves up-regulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway, J. Neurosci., 24, 1350–1357.
McLaughlin, B., Hartnett, K. A., Erhardt, J. A., Legos, J. J., White, R. F., Barone, F. C., and Aizenman, E. (2003) Caspase 3 activation is essential for neuroprotection in preconditioning, Proc. Natl. Acad. Sci. USA, 100, 715–720.
Hoyte, L. C., Papadakis, M., Barber, P. A., and Buchan, A. M. (2006) Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning, Brain Res., 1121, 231–237.
Vlasov, T. D., Korzhevskii, D. E., and Polyakova, E. A. (2005) Ischemic preconditioning of the rat brain as a method of endothelial protection from ischemic/repercussion injury, Neurosci. Behav. Physiol., 35, 567–572.
Nakamura, H., Katsumata, T., Nishiyama, Y., Otori, T., Katsura, K., and Katayama, Y. (2006) Effect of ischemic preconditioning on cerebral blood flow after subsequent lethal ischemia in gerbils, Life Sci., 78, 1713–1719.
Hashiguchi, A., Yano, S., Morioka, M., Hamada, J., Ushio, Y., and Takeuchi, Y. (2004) Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus, J. Cereb. Blood Flow Metab., 24, 271–279.
Cho, S., Park, E. M., Zhou, P., Frys, K., Ross, M. E., and Iadecola, C. (2005) Obligatory role of inducible nitric oxide synthase in ischemic preconditioning, J. Cereb. Blood Flow Metab., 25, 493–501.
Atochin, D. N., Clark, J., Demchenko, I. T., Moskowitz, M. A., and Huang, P. L. (2003) Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases, Stroke, 34, 1299–1303.
Tokuno, S., Chen, F., Pernow, J., Jiang, J., and Valen, G. (2002) Effects of spontaneous or induced brain ischemia on vessel reactivity: the role of inducible nitric oxide synthase, Life Sci., 71, 679–692.
Rybnikova, E., Gluschenko, T., Tulkova, E., Churilova, A., Jaroshevich, O., Baranova, K., and Samoilov, M. (2008) Preconditioning induces prolonged expression of transcription factors pCREB and NF-kappa B in the neocortex of rats before and following severe hypobaric hypoxia, J. Neurochem., 106, 1450–1458.
Stroev, S. A., Tjulkova, E. I., Tugoy, I. A., Gluschenko, T. S., Samoilov, M. O., and Pelto-Huikko, M. (2007) Effects of preconditioning by mild hypobaric hypoxia on the expression of manganese superoxide dismutase in the rat hippocampus, Neurochem. J., 1, 312–317.
Del Zoppo, G. J., Becker, K. J., and Hallenbeck, J. M. (2001) Inflammation after stroke: is it harmful? Arch. Neurol., 58, 669–672.
Bowen, K. K., Naylor, M., and Vemuganti, R. (2006) Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia, Neurochem. Int., 49, 127–135.
Carr-White, G., Koh, T., DeSouza, A., Haxby, E., Kemp, M., and Hooper, J. (2004) Chronic stable ischemia protects against myocyte damage during beating heart coronary surgery, Eur. J. Cardiothorac. Surg., 25, 772–778.
Hazell, A. S. (2007) Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies, Neurochem. Int., 50, 941–953.
Liu, Y. X., Zhang, M., Liu, L. Z., Cui, X., Hu, Y. Y., and Li, W. B. (2012) The role of glutamate transporter-1a in the induction of brain ischemic tolerance in rats, Glia, 60, 112–124.
Zhang, M., Li, W. B., Geng, J. X., Li, Q. J., Sun, X. C., and Xian, X. H. (2007) The upregulation of glial glutamate transporter-1 participates in the induction of brain ischemic tolerance in rats, J. Cereb. Blood Flow Metab., 27, 1352–1368.
Rochelle, D., and Schwartz-Bloom, R. S. (2001) Gamma-aminobutyric acid a neurotransmission and cerebral ischemia, J. Neurochem., 77, 353–371.
Dave, K. R., Lange-Asschenfeldt, C., Raval, A. P., Prado, R., Busto, R., and Saul, I. (2005) Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis, J. Neurosci. Res., 82, 665–673.
Dave, K. R., DeFazio, R. A., Raval, A. P., Torraco, A., Saul, I., and Barrientos, A. (2008) Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon, J. Neurosci., 28, 4172–4182.
Dave, K. R., Saul, I., Busto, R., Ginsberg, M. D., Sick, T. J., and Perez-Pinzon, M. A. (2001) Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus, J. Cereb. Blood Flow Metab., 21, 1401–1410.
Waldenstrom, A., Haney, M., Biber, B., Kavianipour, M., Moritz, T., and Stranden, P. (2010) Ischemic preconditioning is related to decreasing levels of extracellular adenosine that may be metabolically useful in the at-risk myocardium: an experimental study in the pig, Acta Physiol. (Oxford), 199, 1–9.
Yoneda, T., Benedetti, C., Urano, F., Clark, S. G., Harding, H. P., and Ron, D. (2004) Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones, J. Cell Sci., 117, 4055–4066.
DeGracia, D. J., and Hu, B. R. (2007) Irreversible translation arrest in the reperfused brain, J. Cereb. Blood Flow Metab., 27, 875–893.
Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., and Dephoure, N. (2003) Global analysis of protein expression in yeast, Nature, 425, 737–741.
Hebert, D. N., and Molinari, M. (2007) In and out of the ER: protein folding, quality control, degradation, and related human diseases, Physiol. Rev., 8, 1377–1408.
Liu, C., Chen, S., Kamme, F., and Hu, B. R. (2005) Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia, Neuroscience, 134, 69–80.
Balduini, W., Carloni, S., and Buonocore, G. (2009) Autophagy in hypoxia-ischemia induced brain injury: evidence and speculations, Autophagy, 5, 221–223.
Shintani, T., and Klionsky, D. J. (2004) Autophagy in health and disease: a double-edged sword, Science, 306, 990–995.
Park, H. K., Chu, K., Jung, K. H., Lee, S. T., Bahn, J. J., and Kim, M. (2009) Autophagy is involved in the ischemic preconditioning, Neurosci. Lett., 451, 16–19.
McStay, G. P., Salvesen, G. S., and Green, D. R. (2008) Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways, Cell Death Differ., 15, 322–331.
Salvesen, G. S. (2002) Caspases and apoptosis, Essays Biochem., 38, 9–19.
Troy, C. M., and Salvesen, G. S. (2002) Caspases on the brain, J. Neurosci. Res., 69, 145–150.
Namura, S., Zhu, J., Fink, K., Endres, M., Srinivasan, A., Tomaselli, K. J., Yuan, J., and Moskowitz, M. A. (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia, J. Neurosci., 18, 3659–3668.
Clark, R. S., Kochanek, P. M., Chen, M., Watkins, S. C., Marion, D. W., Chen, J., Hamilton, R. L., Loeffert, J. E., and Graham, S. H. (1999) Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury, FASEB J., 13, 813–821.
Kuida, K., Zheng, T. S., Na, S., Kuan, C., Yang, D., Karasuyama, H., Rakic, P., and Flavell, R. A. (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice, Nature, 384, 368–372.
Khalil, H., Peltzer, N., Walicki, J., Yang, J. Y., Dubuis, G., Gardiol, N., Held, W., Bigliardi, P., Marsland, B., Liaudet, L., and Widmann, C. (2012) Caspase-3 protects stressed organs against cell death, Mol. Cell Biol., 32, 4523–4533.
Yang, J. Y., Michod, D., Walicki, J., Murphy, B. M., Kasibhatla, S., Martin, S. J., and Widmann, C. (2004) Partial cleavage of RasGAP by caspases is required for cell survival in mild stress conditions, Mol. Cell Biol., 24, 10425–10436.
Launay, S., Hermine, O., Fontenay, M., Kroemer, G., Solary, E., and Garrido, C. (2005) Vital functions for lethal caspases, Oncogene, 24, 5137–5148.
Cowan, K. N., Leung, W. C., Mar, C., Bhattacharjee, R., Zhu, Y., and Rabinovitch, M. (2005) Caspases from apoptotic myocytes degrade extracellular matrix: a novel remodeling paradigm, FASEB J., 19, 1848–1850.
Krebs, J. F., Srinivasan, A., Wong, A. M., Tomaselli, K. J., Fritz, L. C., and Wu, J. C. (2000) Heavy membrane-associated caspase 3: identification, isolation, and characterization, Biochemistry, 39, 16056–16063.
Denault, J. B., and Salvesen, G. S. (2003) Human caspase-7 activity and regulation by its N-terminal peptide, J. Biol. Chem., 278, 34042–34050.
Pelletier, M., Cartron, P. F., Delaval, F., Meflah, K., Vallette, F. M., and Oliver, L. (2004) Caspase-3 activation is controlled by a sequence located in the N-terminus of its large subunit, Biochem. Biophys. Res. Commun., 316, 93–99.
Ditzel, M., Broemer, M., Tenev, T., Bolduc, C., Lee, T. V., Rigbolt, K. T., Elliott, R., Zvelebil, M., Blagoev, B., Bergmann, A., and Meier, P. (2008) Inactivation of effector caspases through nondegradative polyubiquitinylation, Mol. Cell, 32, 540–553.
Pelletier, M., Oliver, L., Meflah, K., and Vallette, F. M. (2005) Caspase-3 can be pseudo-activated by a Ca2+-dependent proteolysis at a non-canonical site, FEBS Lett., 579, 2364–2368.
Faleiro, L., Kobayashi, R., Fearnhead, H., and Lazebnik, Y. (1997) Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells, EMBO J., 16, 2271–2281.
Miller, M. A., Karacay, B., Zhu, X., O’Dorisio, M. S., and Sandler, A. D. (2006) Caspase 8L, a novel inhibitory isoform of caspase 8, is associated with undifferentiated neuroblastoma, Apoptosis, 11, 15–24.
Beaujouin, M., Baghdiguian, S., Glondu-Lassis, M., Berchem, G., and Liaudet-Coopman, E. (2006) Overexpression of both catalytically active and inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity, Oncogene, 25, 1967–1973.
Levine, B., and Kroemer, G. (2008) Autophagy in the pathogenesis of disease, Cell, 132, 27–42.
Parkhitko, A. A., Favorova, O. O., and Henske, E. P. (2013) Autophagy: mechanisms, regulation and its role in tumorigenesis, Biochemistry (Moscow), 78, 355–367.
Ossovskaya, V. S., and Bunnett, N. W. (2004) Proteaseactivated receptors: contribution to physiology and disease, Physiol. Rev., 84, 579–621.
Vu, T. K., Hung, D. T., Wheaton, V. I., and Coughlin, S. R. (1991) Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation, Cell, 64, 1057–1068.
Bohm, S. K., Khitin, L. M., Grady, E. F., Aponte, G., Payan, D. G., and Bunnett, N. W. (1996) Mechanisms of desensitization and resensitization of proteinase-activated receptor-2, J. Biol. Chem., 271, 22003–22016.
Luo, W., Wang, Y., and Reiser, G. (2007) Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection, Brain Res. Rev., 56, 331–345.
Striggow, F., Riek-Burchardt, M., Kiesel, A., Schmidt, W., Henrich-Noack, P., Breder, J., Krug, M., Reymann, K. G., and Reiser, G. (2001) Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia, Eur. J. Neurosci., 14, 595–608.
Davydova, O. N., Yakovlev, A. A., Lyzhin, A. A., Khaspekov, L. G., and Gulyaeva, N. V. (2010) Deprivation of growth factors leads to specific increase in expression of the PAR-2 receptor mRNA in primary cell cultures of cerebellum, Neirokhimiya, 27, 309–314.
Yakovlev, A. A., Kvichansky, A. A., Lyzhin, A. A., Khaspekov, L. G., and Gulyaeva, N. V. (2013) Glutamate treatment and preconditioning differently affect cathepsin B release and intracellular proteases in primary cultures of cerebellar granular cells, Neurochem. J., 30, 117–127.
Jin, G., Hayashi, T., Kawagoe, J., Takizawa, T., Nagata, T., Nagano, I., Syoji, M., and Abe, K. (2005) Deficiency of PAR-2 gene increases acute focal ischemic brain injury, J. Cereb. Blood Flow Metab., 25, 302–313.
Database MEROPS (http://merops.sanger.ac.uk/).
Schilling, O., and Overall, C. M. (2008) Proteome-derived, database-searchable peptide libraries for identifying protease cleavage sites, Nature Biotechnol., 26, 685–694.
Verspurten, J., Gevaert, K., Declercq, W., and Vandenabeele, P. (2009) Site predicting the cleavage of proteinase substrates, Trends Biochem. Sci., 34, 319–323.
http://www.dmbr.ugent.be/prx/bioit2-public/SitePrediction/index.php
Onufriev, M. V., Yakovlev, A. A., Lyzhin, A. A., Stepanichev, M. Yu., Khaspekov, L. G., and Gulyaeva, N. V. (2009) A secreted caspase-3-substrate-cleaving activity at low pH belongs to cathepsin B: a study on primary brain cell cultures, Biochemistry (Moscow), 74, 281–287.
Yakovlev, A. A., Gorokhovatsky, A. Yu., Onufriev, M. V., Beletsky, I. P., and Gulyaeva, N. V. (2008) Brain cathepsin B cleaves a caspase substrate, Biochemistry (Moscow), 73, 332–336.
Mort, J. S., and Recklies, A. D. (1986) Interrelationship of active and latent secreted human cathepsin B precursors, Biochem. J., 233, 57–63.
Pietras, R. J., and Roberts, J. A. (1981) Cathepsin B-like enzymes. Subcellular distribution and properties in neoplastic and control cells from human ectocervix, J. Biol. Chem., 256, 8536–8544.
Sloane, B. F., Moin, K., Sameni, M., Tait, L. R., Rozhin, J., and Ziegler, G. (1994) Membrane association of cathepsin B can be induced by transfection of human breast epithelial cells with c-Ha-ras oncogene, J. Cell Sci., 107, 373–384.
Guinec, N., Dalet-Fumeron, V., and Pagano, M. (1992) Quantitative study of the binding of cysteine proteinases to basement membranes, FEBS Lett., 308, 305–308.
Barrett, A. J., and Kirschke, H. (1981) Cathepsin B, cathepsin H, and cathepsin L, Methods Enzymol., 80, 535–561.
Rowan, A. D., Feng, R., Konishi, Y., and Mort, J. S. (1993) Demonstration by electrospray mass spectrometry that the peptidyl dipeptidase activity of cathepsin B is capable of rat cathepsin B C-terminal processing, Biochem. J., 294, 923–927.
Pohl, J., Davinic, S., Blaha, I., Strop, P., and Kostka, V. (1987) Chromophoric and fluorophoric peptide substrates cleaved through the dipeptidyl carboxypeptidase activity of cathepsin B, Analyt. Biochem., 165, 96–101.
Polgar, L., and Csoma, C. (1987) Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B, J. Biol. Chem., 262, 14448–14453.
Takahashi, T., Dehdarani, A. H., Yonezawa, S., and Tang, J. (1986) Porcine spleen cathepsin B is an exopeptidase, J. Biol. Chem., 261, 9375–9381.
Khouri, H. E., Plouffe, C., Hasnain, S., Hirama, T., Storer, A. C., and Menard, R. (1991) A model to explain the pH-dependent specificity of cathepsin B-catalyzed hydrolyses, Biochem. J., 275, 751–757.
Willenbrock, F., and Brocklehurst, K. (1985) A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp158 and -161 (papain and actinidin), Gly196 (cathepsin B) and Asn165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alphabenzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalyzed by cathepsin B and of L-arginine 2-naphthylamide catalyzed by cathepsin H, Biochem. J., 227, 521–528.
Turk, B., Bieth, J. G., Bjork, I., Dolenc, I., Turk, D., Cimerman, N., Kos, J., Colic, A., Stoka, V., and Turk, V. (1995) Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins, Biol. Chem. Hoppe Seyler, 376, 225–230.
Afkhami-Goli, A., Noorbakhsh, F., Keller, A. J., Vergnolle, N., Westaway, D., Jhamandas, J. H., Andrade-Gordon, P., Hollenberg, M. D., Arab, H., Dyck, R. H., and Power, C. (2007) Proteinase-activated receptor-2 exerts protective and pathogenic cell type-specific effects in Alzheimer’s disease, J. Immunol., 179, 5493–5503.
Khan, M. Y., Agarwal, S. K., and Ahmad, S. (1992) Structure-activity relationship in buffalo spleen cathepsin B, J. Biochem., 111, 732–735.
Medina, D. L., Fraldi, A., Bouche, V., Annunziata, F., Mansueto, G., Spampanato, C., Puri, C., Pignata, A., Martina, J. A., Sardiello, M., Palmieri, M., Polishchuk, R., Puertollano, R., and Ballabio, A. (2011) Transcriptional activation of lysosomal exocytosis promotes cellular clearance, Dev. Cell, 21, 421–430.
Jaiswal, J. K., Andrews, N. W., and Simon, S. M. (2002) Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells, Cell Biol., 159, 625–635.
Li, D., Ropert, N., Koulakoff, A., Giaume, C., and Oheim, M. (2008) Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes, J. Neurosci., 28, 7648–7658.
Reddy, A., Caler, E. V., and Andrews, N. W. (2001) Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes, Cell, 106, 157–169.
Annunziata, I., Patterson, A., Helton, D., Hu, H., Moshiach, S., Gomero, E., Nixon, R., and d’Azzo, A. (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis, Nature Commun., 4, 2734.
Dou, Y., Wu, H. J., Li, H. Q., Qin, S., Wang, Y. E., Li, J., Lou, H. F., Chen, Z., Li, X. M., Luo, Q. M., and Duan, S. (2012) Microglial migration mediated by ATP-induced ATP release from lysosomes, Cell Res., 22, 1022–1033.
Chen, G., Zhang, Z., Wei, Z., Cheng, Q., Li, X., Li, W., Duan, S., and Gu, X. (2012) Lysosomal exocytosis in Schwann cells contributes to axon remyelination, Glia, 60, 295–305.
Papadakis, M., Hadley, G., Xilouri, M., Hoyte, L. C., Nagel, S., McMenamin, M. M., Tsaknakis, G., Watt, S. M., Drakesmith, C. W., Chen, R., Wood. M. J., Zhao, Z., Kessler, B., Vekrellis, K., and Buchan, A. M. (2013) Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy, Nature Med., 19, 351–357.
Cavallo-Medved, D., Dosescu, J., Linebaugh, B. E., Sameni, M., Rudy, D., and Sloane, B. F. (2003) Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells, Neoplasia, 5, 507–519.
Almeida, P. C., Nantes, I. L., Chagas, J. R., Rizzi, C. C., Faljoni-Alario, A., Carmona, E., Juliano, L., Nader, H. B., and Tersariol, I. L. (2001) Cathepsin B activity regulation. Heparin-like glycosaminoglycans protect human cathepsin B from alkaline pH-induced inactivation, J. Biol. Chem., 276, 944–951.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. A. Yakovlev, N. V. Gulyaeva, 2015, published in Biokhimiya, 2015, Vol. 80, No. 2, pp. 204–213.
Rights and permissions
About this article
Cite this article
Yakovlev, A.A., Gulyaeva, N.V. Possible role of proteases in preconditioning of brain cells to pathological conditions. Biochemistry Moscow 80, 163–171 (2015). https://doi.org/10.1134/S0006297915020030
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915020030