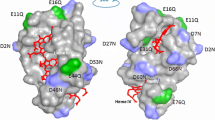
Abstract
Bacteria utilizing insoluble mineral forms of metal oxides as electron acceptors in respiratory processes are widespread in the nature. The electron transfer from a pool of reduced quinones in the cytoplasmic membrane across the periplasm to the bacterial outer membrane and then to an extracellular acceptor is a key step in bacterial dissimilatory metal reduction. Multiheme cytochromes c play a crucial role in the extracellular electron transfer. The bacterium Shewanella oneidensis MR-1 was used as a model organism to study the mechanism of extracellular electron transport. In this review, we discuss recent data on the composition, structures, and functions of multiheme cytochromes c and their functional complexes responsible for extracellular electron transport in Shewanella oneidensis.
Similar content being viewed by others
Abbreviations
- BDMR:
-
bacterial dissimilatory metal reduction
- ETC:
-
electron transport chain
- Fe-NTA:
-
iron nitrilotriacetate
- OMC:
-
outer-membrane cytochrome c
- rmsd:
-
root-meansquare deviation
References
Balashova, V. V., and Zavarzin, G. A. (1980) Anaerobic reduction of ferric iron by hydrogen bacteria, Microbiology, 48, 635–639.
Lovley, D. R., and Phillips, E. J. (1987) Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments, Appl. Environ. Microbiol., 53, 2636–2641.
Lovley, D. R., and Phillips, E. J. (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese, Appl. Environ. Microbiol., 54, 1472–1480.
Gralnick, J. A., and Newman, D. K. (2007) Extracellular respiration, Mol. Microbiol., 65, 1–11.
Chiorse, W. C. (1984) Biology of iron-depositing and manganese-depositing bacteria, Ann. Rev. Microbiol., 38, 515–550.
Myers, C. R., and Nealson, K. H. (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor, Science, 240, 1319–1321.
Lovley, D. R. (1991) Dissimilatory Fe(III) and Mn(IV) reduction, Microbiol. Rev., 55, 259–287.
Lovley, D. R. (1993) Dissimilatory metalloreduction, Ann. Rev. Microbiol., 47, 263–290.
Nealson, K. H., and Saffarini, D. (1994) Iron and manganese in anaerobic respiration environmental significance, physiology, and regulation, Ann. Rev. Microbiol., 48, 311–343.
Weber, K. A., Achenbach, L. A., and Coates, J. D. (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction, Nature Rev. Microbiol., 4, 752–764.
Richardson, D. J., Fredrickson, J. K., and Zachara, J. M. (2012) Electron transport at the microbe-mineral interface: a synthesis of current research challenges, Biochem. Soc. Transact., 40, 1163–1166.
Thamdrup, B., Rossello-Mora, R., and Amann, R. (2000) Microbial manganese and sulfate reduction in Black Sea shelf sediments, Appl. Environ. Microbiol., 66, 2888–2897.
Walker, J. C. (1987) Was the Archaean biosphere upside down? Nature, 329, 710–712.
Lovley, D. R., Holmes, D. E., and Nevin, K. P. (2004) Dissimilatory Fe(III) and Mn(IV) reduction, Adv. Microb. Physiol., 49, 219–286.
Lovley, D. R., Ueki, T., Zhang, T., Malvankar, N. S., Shrestha, P. M., Flanagan, K. A., Aklujkar, M., Butler, J. E., Giloteaux, L., Rotaru, A. E., Holmes, D. E., Franks, A. E., Orellana, R., Risso, C., and Nevin, K. P. (2011) Geobacter: the microbe electric’s physiology, ecology, and practical applications, Adv. Microb. Physiol., 59, 1–100.
Lovley, D. R. (2012) Electromicrobiology, Ann. Rev. Microbiol., 66, 391–409.
Logan, B. E. (2009) Exoelectrogenic bacteria that power microbial fuel cells, Nature Rev. Microbiol., 7, 375–381.
Logan, B. E., and Regan, J. M. (2006) Electricity-producing bacterial communities in microbial fuel cells, Trends Microbiol., 14, 512–518.
Logan, B. E., and Regan, J. M. (2006) Microbial fuel cells — challenges and applications, Environ. Sci. Technol., 40, 5172–5180.
Malvankar, N. S., and Lovley, D. R. (2014) Microbial nanowires for bioenergy applications, Curr. Opin. Biotechnol., 27, 88–95.
Williams, K. H., Bargar, J. R., Lloyd, J. R., and Lovley, D. R. (2013) Bioremediation of uranium-contaminated groundwater: a systems approach to subsurface biogeochemistry, Curr. Opin. Biotechnol., 24, 489–497.
Cutting, R. S., Coker, V. S., Telling, N. D., Kimber, R. L., Pearce, C. I., Ellis, B. L., Lawson, R. S., van der Laan, G., Pattrick, R. A., Vaughan, D. J., Arenholz, E., and Lloyd, J. R. (2010) Optimizing Cr(VI) and Tc(VII) remediation through nanoscale biomineral engineering, Environ. Sci. Technol., 44, 2577–2584.
Jiao, Y., Qian, F., Li, Y., Wang, G., Saltikov, C. W., and Gralnick, J. A. (2011) Deciphering the electron transport pathway for graphene oxide reduction by Shewanella oneidensis MR-1, J. Bacteriol., 193, 3662–3665.
Fredrickson, J. K., Kostandarithes, H. M., Li, S. W., Plymale, A. E., and Daly, M. J. (2000) Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1, Appl. Environ. Microbiol., 66, 2006–2011.
Slobodkin, A. I. (2005) Thermophilic microbial metal reduction, Mikrobiologiya, 74, 581–595.
Wrighton, K. C., Agbo, P., Warnecke, F., Weber, K. A., Brodie, E. L., DeSantis, T. Z., Hugenholtz, P., Andersen, G. L., and Coates, J. D. (2008) A novel ecological role of the Firmicutes identified in thermophilic microbial fuel cells, ISME J., 2, 1146–1156.
Iavarone, A. T., Gorur, A., Yeo, B. S., Tran, R., Melnyk, R. A., Mathies, R. A., Auer, M., Coates, J. D., and Carlson, H. K. (2012) Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria, Proc. Natl. Acad. Sci. USA, 109, 1702–1707.
Ibrahim, A. S., El-Tayeb, M. A., Elbadawi, Y. B., Al-Salamah, A. A., and Antranikian, G. (2012) Hexavalent chromate reduction by alkaliphilic Amphibacillus sp. KSUCr3 is mediated by copper-dependent membrane-associated Cr(VI) reductase, Extremophiles, 16, 659–668.
Gavrilov, S. N., Lloyd, J. R., Kostrikina, N. A., and Slobodkin, A. I. (2012) Fe(III) oxide reduction by a gram-positive thermophile: physiological mechanisms for dissimilatory reduction of poorly crystalline Fe(III) oxide by a thermophilic gram-positive bacterium Carboxydothermus ferrireducens, Geomicrobiol. J., 29, 804–819.
Nevin, K. P., and Lovley, D. R. (2002) Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans, Appl. Environ. Microbiol., 68, 2294–2299.
Myers, J. M., and Myers, C. R. (2001) Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide, Appl. Environ. Microbiol., 67, 260–269.
Beliaev, A. S., and Saffarini, D. A. (1998) Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction, J. Bacteriol., 180, 6292–6297.
Yang, Y., Chen, J., Qiu, D., and Zhou, J. (2013) Roles of UndA and MtrC of Shewanella putrefaciens W3-18-1 in iron reduction, BMC Microbiol., 13, 267; doi: 10.1186/1471-2180-13-267.
Smith, J. A., Lovley, D. R., and Tremblay, P. L. (2013) Outer cell surface components essential for Fe(III) oxide reduction by Geobacter metallireducens, Appl. Environ. Microbiol., 79, 901–907.
Nissen, S., Liu, X., Chourey, K., Hettich, R. L., Wagner, D. D., Pfiffner, S. M., and Loffler, F. E. (2012) Comparative c-type cytochrome expression analysis in Shewanella oneidensis strain MR-1 and Anaeromyxobacter dehalogenans strain 2CP-C grown with soluble and insoluble oxidized metal electron acceptors, Biochem. Soc. Transact., 40, 1204–1210.
Fredrickson, J. K., and Zachara, J. M. (2008) Electron transfer at the microbe-mineral interface: a grand challenge in biogeochemistry, Geobiology, 6, 245–253.
Richter, K., Schicklberger, M., and Gescher, J. (2012) Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration, Appl. Environ. Microbiol., 78, 913–921.
Coursolle, D., and Gralnick, J. A. (2012) Reconstruction of extracellular respiratory pathways for iron(III) reduction in Shewanella oneidensis strain MR-1, Front. Microbiol., 3, 56; doi: 10.3389/fmicb.2012.00056.
Shi, L., Squier, T. C., Zachara, J. M., and Fredrickson, J. K. (2007) Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multiheme c-type cytochromes, Mol. Microbiol., 65, 12–20.
Hartshorne, R. S., Reardon, C. L., Ross, D., Nuester, J., Clarke, T. A., Gates, A. J., Mills, P. C., Fredrickson, J. K., Zachara, J. M., Shi, L., Beliaev, A. S., Marshall, M. J., Tien, M., Brantley, S., Butt, J. N., and Richardson, D. J. (2009) Characterization of an electron conduit between bacteria and the extracellular environment, Proc. Natl. Acad. Sci. USA, 106, 22169–22174.
Richardson, D. J., Edwards, M. J., White, G. F., Baiden, N., Hartshorne, R. S., Fredrickson, J., Shi, L., Zachara, J., Gates, A. J., Butt, J. N., and Clarke, T. A. (2012) Exploring the biochemistry at the extracellular redox frontier of bacterial mineral Fe(III) respiration, Biochem. Soc. Transact., 40, 493–500.
Shi, L., Rosso, K. M., Clarke, T. A., Richardson, D. J., Zachara, J. M., and Fredrickson, J. K. (2012) Molecular underpinnings of Fe(III) oxide reduction by Shewanella oneidensis MR-1, Front. Microbiol., 3, 50; doi: 10.3389/fmicb.2012.00050.
Coursolle, D., and Gralnick, J. A. (2010) Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1, Mol. Microbiol., 77, 995–1008.
Paquete, C. M., and Louro, R. O. (2010) Molecular details of multielectron transfer: the case of multiheme cytochromes from metal respiring organisms, Dalton Transact., 39, 4259–4266.
Moser, C. C., Chobot, S. E., Page, C. C., and Dutton, P. L. (2008) Distance metrics for heme protein electron tunneling, Biochim. Biophys. Acta, 1777, 1032–1037.
Childers, S. E., Ciufo, S., and Lovley, D. R. (2002) Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis, Nature, 416, 767–769.
Harris, H. W., El-Naggar, M. Y., Bretschger, O., Ward, M. J., Romine, M. F., Obraztsova, A. Y., and Nealson, K. H. (2010) Electrokinesis is a microbial behavior that requires extracellular electron transport, Proc. Natl. Acad. Sci. USA, 107, 326–331.
Harris, H. W., El-Naggar, M. Y., and Nealson, K. H. (2012) Shewanella oneidensis MR-1 chemotaxis proteins and electron-transport chain components essential for congregation near insoluble electron acceptors, Biochem. Soc. Transact., 40, 1167–1177.
Lies, D. P., Hernandez, M. E., Kappler, A., Mielke, R. E., Gralnick, J. A., and Newman, D. K. (2005) Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms, Appl. Environ. Microbiol., 71, 4414–4426.
Newman, D. K., and Kolter, R. (2000) A role for excreted quinones in extracellular electron transfer, Nature, 405, 93–97.
Von Canstein, H., Ogawa, J., Shimizu, S., and Lloyd, J. R. (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer, Appl. Environ. Microbiol., 74, 615–623.
Marsili, E., Baron, D. B., Shikhare, I. D., Coursolle, D., Gralnick, J. A., and Bond, D. R. (2008) Shewanella secretes flavins that mediate extracellular electron transfer, Proc. Natl. Acad. Sci. USA, 105, 3968–3973.
Kotloski, N. J., and Gralnick, J. A. (2013) Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis, mBio, 4, e00553–12; doi: 10.1128/mBio.00553-12.
Coursolle, D., Baron, D. B., Bond, D. R., and Gralnick, J. A. (2010) The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis, J. Bacteriol., 192, 467–474.
Ross, D. E., Brantley, S. L., and Tien, M. (2009) Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1, Appl. Environ. Microbiol., 75, 5218–5226.
Wu, C., Cheng, Y. Y., Li, B. B., Li, W. W., Li, D. B., and Yu, H. Q. (2013) Electron acceptor dependence of electron shuttle secretion and extracellular electron transfer by Shewanella oneidensis MR-1, Bioresource Technol., 136, 711–714.
Malvankar, N., Vargas, M., Nevin, K. P., Franks, A. E., Leang, C., Kim, B.-C., Inoue, K., Mester, T., Covalla, S. F., Johnson, J. P., Rotello, V. M., Tuominen, M. T., and Lovley, D. R. (2011) Tunable metallic-like conductivity in nanostructured biofilms comprised of microbial nanowires, Nature Nanotechnol., 6, 573–579.
Lovley, D. R. (2012) Long-range electron transport to Fe(III) oxide via pili with metallic-like conductivity, Biochem. Soc. Transact., 40, 1186–1190.
Malvankar, N. S., and Lovley, D. R. (2012) Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics, ChemSusChem, 5, 1039–1046.
Reguera, G., McCarthy, K. D., Mehta, T., Nicoll, J. S., Tuominen, M. T., and Lovley, D. R. (2005) Extracellular electron transfer via microbial nanowires, Nature, 435, 1098–1101.
Lovley, D. R. (2008) Extracellular electron transfer: wires, capacitors, iron lungs, and more, Geobiology, 6, 225–231.
Leang, C., Qian, X., Mester, T., and Lovley, D. R. (2010) Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens, Appl. Environ. Microbiol., 76, 4080–4084.
Lovley, D. R. (2011) Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination, Energy Environ. Sci., 4, 4896–4906.
Esteve-Nunez, A., Sosnik, J., Visconti, P., and Lovley, D. R. (2008) Fluorescent properties of c-type cytochromes reveal their potential role as an extracytoplasmic electron sink in Geobacter sulfurreducens, Environ. Microbiol., 10, 497–505.
Mitchell, A. C., Peterson, L., Reardon, C. L., Reed, S. B., Culley, D. E., Romine, M. R., and Geesey, G. G. (2012) Role of outer membrane c-type cytochromes MtrC and OmcA in Shewanella oneidensis MR-1 cell production, accumulation, and detachment during respiration on hematite, Geobiology, 10, 355–370.
Meyer, T. E., Tsapin, A. I., Vandenberghe, I., de Smet, L., Frishman, D., Nealson, K. H., Cusanovich, M. A., and van Beeumen, J. J. (2004) Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes, Omics, 8, 57–77.
Myers, C. R., and Myers, J. M. (1997) Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1, J. Bacteriol., 179, 1143–1152.
Myers, C. R., and Myers, J. M. (2002) MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1, Appl. Environ. Microbiol., 68, 5585–5594.
Beliaev, A. S., Saffarini, D. A., McLaughlin, J. L., and Hunnicutt, D. (2001) MtrC, an outer membrane decaheme c cytochrome required for metal reduction in Shewanella putrefaciens MR-1, Mol. Microbiol., 39, 722–730.
Pitts, K. E., Dobbin, P. S., Reyes-Ramirez, F., Thomson, A. J., Richardson, D. J., and Seward, H. E. (2003) Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates, J. Biol. Chem., 278, 27758–27765.
Marritt, S. J., McMillan, D. G. G., Shi, L., Fredrickson, J. K., Zachara, J. M., Richardson, D. J., Jeuken, L. J. C., and Butt, J. N. (2012) The roles of CymA in support of the respiratory flexibility of Shewanella oneidensis MR-1, Biochem. Soc. Transact., 40, 1217–1221.
Marritt, S. J., Lowe, T. G., Bye, J., McMillan, D. G. G., Shi, L., Fredrickson, J., Zachara, J., Richardson, D. J., Cheesman, M. R., Jeuken, L. J. C., and Butt, J. N. (2012) A functional description of CymA, an electron-transfer hub supporting anaerobic respiratory flexibility in Shewanella, Biochem. J., 444, 465–474.
Louro, R. O., and Paquete, C. M. (2012) The quest to achieve the detailed structural and functional characterization of CymA, Biochem. Soc. Transact., 40, 1291–1294.
McMillan, D. G. G., Marritt, S. J., Butt, J. N., and Jeuken, L. J. C. (2012) Menaquinone-7 is specific cofactor in tetraheme quinol dehydrogenase CymA, J. Biol. Chem., 287, 14215–14225.
Zargar, K., and Saltikov, C. W. (2009) Lysine-91 of the tetraheme c-type cytochrome CymA is essential for quinone interaction and arsenate respiration in Shewanella sp. strain ANA-3, Arch. Microbiol., 191, 797–806.
Rodrigues, M. L., Scott, K. A., Sansom, M. S. P., Pereira, I. A. C., and Archer, M. (2008) Quinol oxidation by c-type cytochromes: structural characterization of the menaquinol binding site of NrfHA, J. Mol. Biol., 381, 341–350.
Bewley, K. D., Ellis, K. E., Firer-Sherwood, M. A., and Elliott, S. J. (2013) Multi-heme proteins: nature’s electronic multi-purpose tool, Biochim. Biophys. Acta, 1827, 938–948.
Myers, J. M., and Myers, C. R. (2000) Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone, J. Bacteriol., 182, 67–75.
Schwalb, C., Chapman, S. K., and Reid, G. A. (2003) The tetraheme cytochrome CymA is required for anaerobic respiration with dimethyl sulfoxide and nitrite in Shewanella oneidensis, Biochemistry, 42, 9491–9497.
Gao, H. C., Yang, Z. K., Barua, S., Reed, S. B., Romine, M. F., Nealson, K. H., Fredrickson, J. K., Tiedje, J. M., and Zhou, J. Z. (2009) Reduction of nitrate in Shewanella oneidensis depends on atypical NAP and NRF systems with NapB as a preferred electron transport protein from CymA to NapA, ISME J., 3, 966–976.
Gao, H., Barua, S., Liang, Y., Wu, L., Dong, Y., Reed, S., Chen, J., Culley, D., Kennedy, D., Yang, Y., He, Z., Nealson, K. H., Fredrickson, J. K., Tiedje, J. M., Romine, M., and Zhou, J. (2010) Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration, Microb. Biotechnol., 3, 455–466.
Field, S. J., Dobbin, P. S., Cheesman, M. R., Watmough, N. J., Thomson, A. J., and Richardson, D. J. (2000) Purification and magneto-optical spectroscopic characterization of cytoplasmic membrane and outer membrane multiheme c-type cytochromes from Shewanella frigidimarina NCIMB400, J. Biol. Chem., 275, 8515–8522.
Schwalb, C., Chapman, S. K., and Reid, G. A. (2002) The membrane-bound tetraheme c-type cytochrome CymA interacts directly with the soluble fumarate reductase in Shewanella, Biochem. Soc. Transact., 30, 658–662.
Youngblut, M., Judd, E. T., Srajer, V., Sayyed, B., Goelzer, T., Elliott, S. J., Schmidt, M., and Pacheco, A. A. (2012) Laue crystal structure of Shewanella oneidensis cytochrome c nitrite reductase from a high-yield expression system, J. Biol. Inorg. Chem., 17, 647–662.
Bretschger, O., Obraztsova, A., Sturm, C. A., Chang, I. S., Gorby, Y. A., Reed, S. B., Culley, D. E., Reardon, C. L., Barua, S., Romine, M. F., Zhou, J., Beliaev, A. S., Bouhenni, R., Saffarini, D., Mansfeld, F., Kim, B. H., Fredrickson, J. K., and Nealson, K. H. (2007) Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants, Appl. Environ. Microbiol., 73, 7003–7012.
Schuetz, B., Schicklberger, M., Kuermann, J., Spormann, A. M., and Gescher, J. (2009) Periplasmic electron transfer via the c-type cytochromes MtrA and FccA of Shewanella oneidensis MR-1, Appl. Environ. Microbiol., 75, 7789–7796.
Clarke, T. A., Cole, J. A., Richardson, D. J., and Hemmings, A. M. (2007) The crystal structure of the pentaheme c-type cytochrome NrfB and characterization of its solution-state interaction with the pentaheme nitrite reductase NrfA, Biochem. J., 406, 19–30.
Mowat, C. G., and Chapman, S. K. (2005) Multi-heme cytochromes — new structures, new chemistry, Dalton Transact., 21, 3381–3389.
Richardson, D. J., Butt, J. N., Fredrickson, J. K., Zachara, J. M., Shi, L., Edwards, M. J., White, G., Baiden, N., Gates, A. J., Marritt, S. J., and Clarke, T. A. (2012) The “porin-cytochrome” model for microbe-to-mineral electron transfer, Mol. Microbiol., 85, 201–212.
Ross, D. E., Ruebush, S. S., Brantley, S. L., Hartshorne, R. S., Clarke, T. A., Richardson, D. J., and Tien, M. (2007) Characterization of protein-protein interactions involved in iron reduction by Shewanella oneidensis MR-1, Appl. Environ. Microbiol., 73, 5797–5808.
Schicklberger, M., Bucking, C., Schuetz, B., Heide, H., and Gescher, J. (2011) Involvement of the Shewanella oneidensis decaheme cytochrome MtrA in the periplasmic stability of the barrel protein MtrB, Appl. Environ. Microbiol., 77, 1520–1523.
Firer-Sherwood, M. A., Ando, N., Drennan, C. L., and Elliott, S. J. (2011) Solution-based structural analysis of the decaheme cytochrome, MtrA, by small-angle X-ray scattering and analytical ultracentrifugation, J. Phys. Chem. B, 115, 1208–1214.
White, G. F., Shi, Z., Shi, L., Wang, Z., Dohnalkova, A. C., Marshall, M. J., Fredrickson, J. K., Zachara, J. M., Butt, J. N., Richardson, D. J., and Clarke, T. A. (2013) Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals, Proc. Natl. Acad. Sci. USA, 110, 6346–6351.
Gralnick, J. A. (2012) On conducting electron traffic across the periplasm, Biochem. Soc. Transact., 40, 1178–1180.
Taylor, P., Pealing, S. L., Reid, G. A., Chapman, S. K., and Walkinshaw, M. D. (1999) Structural and mechanistic mapping of a unique fumarate reductase, Nature Struct. Biol., 6, 1108–1112.
Leys, D., Tsapin, A. S., Nealson, K. H., Meyer, T. E., Cusanovich, M. A., and Van Beeumen, J. J. (1999) Structure and mechanism of the flavocytochrome c fumarate reductase of Shewanella putrefaciens MR-1, Nature Struct. Biol., 6, 1113–1117.
Pessanha, M., Rothery, E. L., Miles, C. S., Reid, G. A., Chapman, S. K., Louro, R. O., Turner, D. L., Salgueiro, C. A., and Xavier, A. V. (2009) Tuning of functional heme reduction potentials in Shewanella fumarate reductases, Biochim. Biophys. Acta, 1787, 113–120.
Leys, D., Meyer, T. E., Tsapin, A. I., Nealson, K. H., Cusanovich, M. A., and Van Beeumen, J. J. (2002) Crystal structures at atomic resolution reveal the novel concept of “electron-harvesting” as a role for the small tetraheme cytochrome c, J. Biol. Chem., 277, 35703–35711.
Fonseca, B. M., Paquete, C. M., Neto, S. E., Pacheco, I., Soares, C. M., and Louro, R. O. (2013) Mind the gap: cytochrome interactions reveal electron pathways across the periplasm of Shewanella oneidensis MR-1, Biochem. J., 449, 101–108.
Firer-Sherwood, M., Pulcu, G. S., and Elliott, S. J. (2008) Electrochemical interrogations of the Mtr cytochromes from Shewanella: opening a potential window, J. Biol. Inorg. Chem., 13, 849–854.
Schutz, B., Seidel, J., Sturm, G., Einsle, O., and Gescher, J. (2011) Investigation of the electron transport chain to and the catalytic activity of the diheme cytochrome c peroxidase CcpA of Shewanella oneidensis, Appl. Environ. Microbiol., 77, 6172–6180.
Shi, L., Chen, B., Wang, Z., Elias, D. A., Mayer, M. U., Gorby, Y. A., Ni, S., Lower, B. H., Kennedy, D. W., Wunschel, D. S., et al. (2006) Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1, J. Bacteriol., 188, 4705–4714.
Bucking, C., Popp, F., Kerzenmacher, S., and Gescher, J. (2010) Involvement and specificity of Shewanella oneidensis outer membrane cytochromes in the reduction of soluble and solid-phase terminal electron acceptors, FEMS Microbiol. Lett., 306, 144–151.
Myers, J. M., and Myers, C. R. (2001) Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide, Appl. Environ. Microbiol., 67, 260–269.
Clarke, T. A., Edwards, M. J., Gates, A. J., Hall, A., White, G. F., Bradley, J., Reardon, C. L., Shi, L., Beliaev, A. S., Marshall, M. J., Wang, Z., Watmough, N. J., Fredrickson, J. K., Zachara, J. M., Butt, J. N., and Richardson, D. J. (2011) Structure of a bacterial cell surface decaheme electron conduit, Proc. Natl. Acad. Sci. USA, 108, 9384–9389.
Hartshorne, R. S., Jepson, B. N., Clarke, T. A., Field, S. J., Fredrickson, J., Zachara, J., Shi, L., Butt, J. N., and Richardson, D. J. (2007) Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors, J. Biol. Inorg. Chem., 12, 1083–1094.
Lower, B. H., Shi, L., Yongsunthon, R., Droubay, T. C., McCready, D. E., and Lower, S. K. (2007) Specific bonds between an iron oxide surface and outer membrane cytochromes MtrC and OmcA from Shewanella oneidensis MR-1, J. Bacteriol., 189, 4944–4952.
Eggleston, C. M., Voros, J., Shi, L., Lower, B. H., Droubay, T. C., and Colberg, P. J. S. (2008) Binding and direct electrochemistry of OmcA, an outer membrane cytochrome from an iron reducing bacterium, with oxide electrodes: a candidate biofuel cell system, Inorg. Chim. Acta, 361, 769–777.
Xiong, Y., Shi, L., Chen, B., Mayer, M. U., Lower, B. H., Londer, Y., Bose, S., Hochella, M. F., Fredrickson, J. K., and Squier, T. C. (2006) High-affinity binding and direct electron transfer to solid metals by the Shewanella oneidensis MR-1 outer membrane c-type cytochrome OmcA, J. Am. Chem. Soc., 128, 13978–13979.
Lower, B. H., Lins, R. D., Oestreicher, Z., Straatsma, T. P., Hochella, M. F., Jr., Shi, L., and Lower, S. K. (2008) In vitro evolution of a peptide with a hematite binding motif that may constitute a natural metal-oxide binding archetype, Environ. Sci. Technol., 42, 3821–3827.
Wigginton, N. S., Rosso, K. M., and Hochella, M. F. (2007) Mechanisms of electron transfer in two decaheme cytochromes from a metal-reducing bacterium, J. Phys. Chem. B, 111, 12857–12864.
Shi, L., Chen, B. W., Wang, Z. M., Elias, D. A., Mayer, M. U., Gorby, Y. A., Ni, S., Lower, B. H., Kennedy, D. W., Wunschel, D. S., et al. (2006) Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1, J. Bacteriol., 188, 4705–4714.
Edwards, M. J., Baiden, N. A., Johs, A., Tomanicek, S. J., Liang, L., Shi, L., Fredrickson, J. K., Zachara, J. M., Gates, A. J., Butt, J. N., Richardson, D. J., and Clarke, T. A. (2014) The X-ray crystal structure of Shewanella oneidensis OmcA reveals new insight at the microbe?mineral interface, FEBS Lett., 588, 1886–1890.
Edwards, M. J., Fredrickson, J. K., Zachara, J. M., Richardson, D. J., and Clarke, T. A. (2012) Analysis of structural MtrC models based on homology with the crystal structure of MtrF, Biochem. Soc. Transact., 40, 1181–1185.
Moser, C. C., Chobot, S. E., Page, C. C., and Dutton, P. L. (2008) Distance metrics for heme protein electron tunneling, Biochim. Biophys. Acta, 1777, 1032–1037.
Edwards, M. J., Hall, A., Shi, L., Fredrickson, J. K., Zachara, J. M., Butt, J. N., Richardson, D. J., and Clarke, T. A. (2012) The crystal structure of the extracellular 11-heme cytochrome UndA reveals a conserved 10-heme motif and defined binding site for soluble iron chelates, Structure, 20, 1275–1284.
Yang, Y., Chen, J., Qiu, D., and Zhou, J. (2013) Roles of UndA and MtrC of Shewanella putrefaciens W3-18-1 in iron reduction, BMC Microbiol., 13, 267.
Breuer, M., Rosso, K. M., and Blumberger, J. (2014) Electron flow in multiheme bacterial cytochromes is a balancing act between heme electronic interaction and redox potentials, Proc. Natl. Acad. Sci. USA, 111, 611–616.
Breuer, M., Zarzycki, P., Blumberger, J., and Rosso, K. M. (2012) Thermodynamics of electron flow in the bacterial decaheme cytochrome MtrF, J. Am. Chem. Soc., 134, 9868–9871.
Breuer, M., Zarzycki, P., Shi, L., Clarke, T. A., Edwards, M. J., Butt, J. N., Richardson, D. J., Fredrickson, J. K., Zachara, J. M., Blumberger, J., and Rosso, K. M. (2012) Molecular structure and free energy landscape for electron transport in the decaheme cytochrome MtrF, Biochem. Soc. Transact., 40, 1198–1203.
Okamoto, A., Hashimoto, K., Nealson, K. H., and Nakamura, R. (2013) Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones, Proc. Natl. Acad. Sci. USA, 110, 7856–7861.
Shi, L., Rosso, K. M., Zachara, J. M., and Fredrickson, J. K. (2012) Mtr extracellular electron-transfer pathways in Fe(III)-reducing or Fe(II)-oxidizing bacteria: a genomic perspective, Biochem. Soc. Transact., 40, 1261–1267.
Bucking, C., Piepenbrock, A., Kappler, A., and Gescher, J. (2012) Outer-membrane cytochrome-independent reduction of extracellular electron acceptors in Shewanella oneidensis, Microbiology, 158, 2144–2157.
Bewley, K. D., Firer-Sherwood, M. A., Mock, J. Y., Ando, N., Drennan, C. L., and Elliott, S. J. (2012) Mind the gap: diversity and reactivity relationships among multiheme cytochromes of the MtrA/DmsE family, Biochem. Soc. Transact., 40, 1268–1273.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T. V. Tikhonova, V. O. Popov, 2014, published in Uspekhi Biologicheskoi Khimii, 2014, Vol. 54, pp. 349–384.
Rights and permissions
About this article
Cite this article
Tikhonova, T.V., Popov, V.O. Structural and functional studies of multiheme cytochromes c involved in extracellular electron transport in bacterial dissimilatory metal reduction. Biochemistry Moscow 79, 1584–1601 (2014). https://doi.org/10.1134/S0006297914130094
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297914130094