Abstract
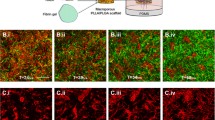
Angiogenic therapies have been designed for many pathological conditions, but when used as a single therapy, the clinical results have fallen short of expectations. In addition, strategies for vascularizing engineered tissues have been unsuccessful in promoting the formation of an extensive, stable vasculature. Recent evidence suggests that mural cells play a critical role in the success of these approaches, but our current understanding of the function of mural cells in the microvasculature is incomplete. We studied the three-dimensional spatial and temporal kinetics of the mural cell markers desmin and smooth muscle alpha actin during angiogenesis in an in vivo fibrin gel model. The results led to the following conclusions: (1) desmin and smooth muscle alpha actin positive cells are present during the initial development of vessel sprouts; (2) the presence of these cells in the microvasculature is not always an indicator of vessel stability; and (3) the mural cell markers desmin and smooth muscle alpha actin exhibit differential staining patterns during vessel formation. These findings shed new light on the complexity of the relationship between mural cells and the formation of a mature, stable microcirculation.
Similar content being viewed by others
REFERENCES
Abramsson, A., O. Berlin, H. Papayan, D. Paulin, M. Shani, and C. Betsholtz. Analysis of mural cell recruitment to tumor vessels. Circulation 105:112–117, 2002.
Benjamin, L. E., D. Golijanin, A. Itin, D. Pode, and E. Keshet. Selective ablation of immature blood vessels in established tumors follows vascular endothelial growth factor withdrawal. J. Clin. Invest. 103:159–165, 1999.
Benjamin, L. E., I. Hemo, and E. Keshet. A plasticity window for blood vessel remodeling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125:1591–1598, 1998.
Bondjers, C., M. Kalen, M. Hellstrom, S. J. Scheidl, A. Abramsson, O. Renner, P. Lindahl, H. Cho, J. Kehrl, and C. Betsholtz. Transcriptional profiling of platelet-derived growth factor-B-deficient mice identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am. J. Pathol. 162:721–729, 2003.
Brey, E. M., T.W. King, C. Johnston, L.V. McIntire, G. P. Reece, and C. W. Patrick, Jr. A technique for quantitative 3D imaging of microvascular structure. Microvasc. Res. 63:279–294, 2002.
Currie, L. J., J. R. Sharpe, and R. Martin. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: A review. Plast. Reconstr. Surg. 108:1713–1726, 2001.
Darland, D. C., and P. D'Amore. Cell-cell interactions in vascular development. Curr. Top. Dev. Biol. 52:107–149, 2001.
Davis, G. E., and C. W. Camarillo. An alpha 2 beta 1 integrindependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 224:39–51, 1996.
Dvorak, H. F., V. S. Harvey, P. Estrella, L. F. Brown, J. McDonagh, and A. M. Dvorak. Fibrin containing gels induce angiogenesis implications for tumor stroma generation and wound healing. Lab. Invest. 57:673–686, 1987.
Egginton, S., and M. Gerritsen. Lumen formation: In vivo versus in vitro observations. Microcirculation 10:45–61. 2003.
Egginton, S., A. L. Zhou, M. D. Brown, and O. Hudlicka. The role of pericytes in controlling angiogenesis in vivo. Adv. Exp. Med. Biol. 476:81–99, 2000.
Frid, M. G.,V. A. Kale, and K. R. Stenmark. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: In vitro analysis. Circ. Res. 90:1189–1196, 2002.
Gee, M. S., W. N. Procopio, S. Makonnen, M. D. Feldman, N.M Yeilding, and W. M. Lee. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am. J. Pathol. 162:183–193, 2003.
Gerritsen, M. E., R. Soriano, S. Yang, C. Zlot, G. Ingle, K. Toy, and P. M. Williams. Branching out: A molecular fingerprint of endothelial differentiation into tube-like structures generated by affymetrix oligonucleotide arrays. Microcirculation 10:63–81, 2003.
Hellstrom, M., M. Kalen, P. Lindalh, A. Abramsson, and C. Betsholtz. Role of PDGF-B and PDGF-βin recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126:3047–3055, 1999.
Hirschi, K. K., and M. A. Goodell. Common origins of blood and blood vessels in adults? Differentiation 68:186–192, 2001.
Hirschi, K. K., S. A. Rohovsky, L. H. Beck, S. R. Smith, and P. A. D'Amore. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 84:298–305, 1999.
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 7:987–989, 2001.
Masaki, I., Y. Yonemitsu, A. Yamashita, S. Sata, M. Tanii, K. Komori, K. Nakagawa, X. Hou, Y. Nagai, M. Hasegawa, K. Sugimachi, and K. Sueishi. Angiogenic gene therapy for experimental critical limb ischemia acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ. Res. 90:966–973, 2002.
Morikawa, S., P. Baluk, T. Kaidoh, A. Haskell, R. K. Jain, and D. M. McDonald. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 160:985–1000, 2000.
Orlidge, A., and P. A. D'Amore. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol. 105:1455–1462, 1987.
Ponce, A. M., and R. J. Price. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc. Res. 65:45–48, 2003.
Powell, D. W., R. C. Mifflin, J. D. Valentich, S. E. Crowe, J. I. Saada, and A. B. West. Myofibroblasts. I. Paracrine cells in health and disease. Am. J. Physiol. (Cell Physiol.) 46:C1–C19, 1999.
Ramsauer, M., J. Kunz, D. Krause, and R. Dermietzel. Regulation of a blood-brain barrier-specific enzyme expressed by cerebral pericytes (pericytic aminopeptidase N/pAPN) under cell culture conditions. J. Cereb. Blood Flow Metab. 18:1270–1281, 1998.
Simper, D., P. G. Stalbeorger, C. J. Panetta, S. Wang, and N. M. Caplice. Smooth muscle progenitor cells in human blood. Circulation 106:1199–1204, 2002.
Skalak, T. C., and R. J. Price. The role of mechanical stress in microvascular remodeling. Microcirculation 3:143–165, 1996.
Sundberg, C., M. Kowanetz, L. F. Brown, M. Detmar, and H. F. Dvorak. Stable expression of angiopoietin-1 and other markers by cultured pericytes: Phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab. Invest. 82:387–401, 2002.
Taniyama, Y., R. Morishita, K. Hiraoka, M. Aoki, H. Nakagami, K. Yamasaki, K. Matsumoto, T. Nakamura, Y. Kaneda, and T. Ogihara. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: Molecular mechanisms of delayed angiogenesis in diabetes. Circulation 104:2344–2350, 2001.
Uemura, A., M. Ogawa, M. Hirashima, T. Fujiwara, S. Koyama, H. Takagi, Y. Honda, S. J. Wiegand, G. D. Yancopoulos, and S. Nishikawa. Recombinant angiopoietin-1 restores higher order architecture of growing vessels in mice in the absence of mural cells. J. Clin. Invest. 110:1619–1628, 2002.
Woods, R. P., S. R. Cherry, and J. C. Mazziotta. Rapid automated algorithm for aligning and reslicingPETimages. J. Comp. Assist. Tomogr. 16:620–633, 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brey, E.M., McIntire, L.V., Johnston, C.M. et al. Three-Dimensional, Quantitative Analysis of Desmin and Smooth Muscle Alpha Actin Expression During Angiogenesis. Annals of Biomedical Engineering 32, 1100–1107 (2004). https://doi.org/10.1114/B:ABME.0000036646.17362.c4
Issue Date:
DOI: https://doi.org/10.1114/B:ABME.0000036646.17362.c4