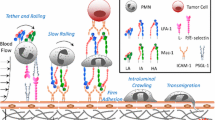
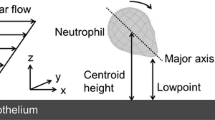
Abstract
Of the white blood cells that traverse the circulation, the polymorphonuclear leukocytes commonly called neutrophils, are the most numerous, numbering ∼5000 per microliter of blood. In a sense, these cells are the emergency response unit within the body's circulatory highway since they are the first to be recruited at sites of tissue trauma, infection, or inflammation. The chief function of neutrophils is the capture, phagocytosis, and degradation of foreign invaders. To carry out this critical function, neutrophils sense infection via chemotactic receptors that trigger cellular activation upon ligation of as few as 10–100 bacterial or chemokine peptides. Despite this acute sensitivity to stimuli, neutrophils circulate in healthy individuals largely in a passive state, with a very low efficiency of capture and arrest on quiescent endothelium. Here, we define the efficiency as the fraction of all cells passing by a given length of vessel wall that is captured and achieves firm adhesion. Within seconds of chemotactic signaling, adhesion molecules expressed on the plasma membrane of neutrophils are activated and a rapid boost in the efficiency of stable adhesion is detected. This occurs for both homotypic neutrophil–neutrophil adhesion and neutrophil capture and adhesion on inflamed endothelium. With such large numbers of circulating neutrophils, and with their inherent capability to rapidly adhere to postcapillary venular endothelium, the body has evolved a diverse set of mechanisms to regulate the inflammatory cascade that begins with intracellular signaling and leads to cell arrest and extravasation at sites of tissue insult. In this article we will focus on the interplay between particle–fluid dynamics that involve the shear and normal forces that transport cells transversely and axially within the vessel, and the activation and interaction of adhesion molecules that involve receptor–ligand bond formation that enables the neutrophils to resist wall shear stress and adhere to specific sites of inflammation. © 2002 Biomedical Engineering Society.
PAC2002: 8719Tt, 8717-d, 8714Ee, 8380Lz
Similar content being viewed by others
REFERENCES
Alon, R., S. Chen, R. Fuhlbrigge, K. D. Puri, and T. A. Springer. The kinetics and shear threshold of transient and rolling interactions of L–selectin with its ligand on leukocytes. Proc. Natl. Acad. Sci. U.S.A. 95:11631–11636, 1998.
Alon, R., T. Feizi, C. T. Yuen, R. C. Fuhlbrigge, and T. A. Springer. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J. Immunol. 154:5356–5366, 1995.
Alon, R., R. C. Fuhlbrigge, E. B. Finger, and T. A. Springer. Interactions through L–selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM–1 in shear flow. J. Cell Biol. 135:849–865, 1996.
Alon, R., D. A. Hammer, and T. A. Springer. Lifetime of the P–selectin–carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature (London) 374:539–542, 1995.
Anderson, D. C., T. K. Kishimoto, and C. W. Smith. Leukocyte adhesion deficiency and other disorders of leukocyte adherence and motility. In: The Metabolic and Molecular Bases of Inherited Disease, edited by C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle. New York: McGraw–Hill, 1995, pp. 3955–3994.
Anderson, D. C., F. C. Schmalstieg, S. Kohl, M. A. Arnaout, B. J. Hughes, M. F. Tosi, G. J. Buffone, B. R. Brinkley, W. D. Dickey, J. S. Abramson, T. A. Springer, L. A. Boxer, J. M. Hollers, and C. W. Smith. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): Common relationship to diminished cell adherence. J. Clin. Invest. 74:536–551, 1984.
Anderson, D. C., and T. A. Springer. Leukocyte adhesion deficiency: An inherited defect in the Mac–1, LFA–1 and p150, 95 glycoproteins. Annu. Rev. Med. 38:175–194, 1987.
Arp, P., and S. G. Mason. Orthokinetic collisions of hard spheres in simple shear flow. Can. J. Chem. 54:3760–3774, 1976.
Atherton, A., and G. V. R. Born. Relationship between the velocity of rolling granulocytes and that of the blood flow in venules. J. Physiol. Paris 233:157–165, 1973.
Bartok, W., and S. G. Mason. Particle motions in sheared suspensions. V. Rigid rods and collision doublets of spheres. J. Colloid Sci. 12:243–262, 1957.
Bell, G. I. Models for the specific adhesion of cells to cells. A theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science 200:618–627, 1978.
Bennett, T. A., C. M. G. Schammel, E. B. Lynam, D. A. Guyer, A. Mellors, B. Edwards, S. Rogelj, and L. A. Sklar. Evidence for a third component in neutrophil aggregation: Potential roles of O–linked glycoproteins as L–selectin counter–structures. J. Leukoc Biol. 58:510–518, 1995.
Bongrand, P., C. Capo, J.–L. Mege, and A.–M. Benoliel. Use of hydrodynamic flows to study cell adhesion. In: Physical Basis of Cell–Cell Adhesion, edited by P. Bongrand. Boca Raton, FL: Chemical Rubber, 1998, pp. 125–156.
Brenner, B., S. Junge, A. Birle, S. Kadel, and O. Linderkamp. Surfactant modulates intracellular signaling of the adhesion receptor L–selectin. Pediatr. Res. 48:283–288, 2000.
Butcher, E. C. Leukocyte–endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell 67:1033–1036, 1991.
Chen, S., and T. A. Springer. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 144:185–200, 1999.
Cokelet, G. R., and H. L. Goldsmith. Decreased hydrodynamic resistance in the two–phase flow of blood through small vertical tubes at low flow rate. Circ. Res. 68:1–17, 1991.
Craddock, P. R., D. E. Hammerschmidt, C. F. Moldow, O. Yamada, and H. S. Jacob. Granulocyte aggregation as a manifestation of membrane interactions with complement. Possible role in leukocyte margination, microvascular occlusion, and endothelial damage. Semin Hematol. 16:140, 1979.
Craddock, P. R., D. E. Hammerschmidt, J. G. White, A. P. Dalmasso, and H. S. Jacob. Complement (C5a)–induced granulocyte aggregation in vitro: A possible mechanism of complement–mediated leukostasis and leukopenia. J. Clin. Invest. 60:260–274, 1977.
Curtis, A. S. G., and L. M. Hocking. Collision efficiency of equal spherical particles in a shear flow. The influence of London–van der Waals forces. Trans. Faraday Soc. 66:1381–1390, 1970.
Doerschuk, C. M., M. F. Allard, and J. C. Hogg. Neutrophil kinetics in rabbits during infusion of zymosan–activated plasma. J. Appl. Physiol. 67:88–95, 1989.
Doerschuk, C. M., R. K. Winn, and J. M. Harlan. Mechanisms of neutrophil emigration. In: Leukocyte Adhesion Molecules: Structure, Function, and Regulation, edited by T. A. Springer, D. C. Anderson, A. S. Rosenthal, and R. Rothlein. New York: Springer, 1989, pp. 87–94.
Dore, M., S. I. Simon, B. J. Hughes, M. L. Entman, and C. W. Smith. P–selectin–and CD18–mediated recruitment of canine neutrophils under conditions of shear stress. Vet. Pathol. 32:258–268, 1995.
Doyle, N. A., S. D. Bhagwan, B. B. Meek, G. J. Kutkoski, D. A. Steeber, T. F. Tedder, and C. M. Doerschuk. Neutrophil margination, sequestration, and emigration in the lungs of L–selectin–deficient mice. J. Clin. Invest. 99:526–533, 1997.
Engler, R. L., M. D. Dahlgren, D. D. Morris, M. A. Peterson, and G. W. Schmid–Schonbein. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am. J. Physiol. 251:H314–H323, 1986.
Eriksson, E. E., X. Xie, J. Werr, P. Thoren, and L. Lindbom. Importance of primary capture and L–selectin–dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J. Exp. Med. 194:205–218, 2001.
Etzioni, A., M. Frydman, S. Pollack, I. Avidor, M. L. Phillips, J. C. Paulson, and R. Gershoni–Barugh. Brief report: Recurrent severe infections caused by a novel leukocyte adhesion deficiency. N. Engl. J. Med. 327:1789–1792, 1992.
Evans, E. Looking inside molecular bonds at biological interfaces with dynamic force spectroscopy. Biophys. Chem. 82:83–97, 1999.
Evans, E., A. Leung, D. Hammer, and S. Simon. Chemically distinct transition states govern rapid dissociation of single L–selectin bonds under force. PNAS 98:3784–3789, 2001.
Evans, E., and K. Ritchie. Dynamic strength of molecular adhesion bonds. Biophys. J. 72:1541–1555, 1997.
Evans, E., and A. Yeung. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 56:151–160, 1989.
Fewell, M. E., and J. D. Hellums. The secondary flow of Newtonian fluids in cone–and plate viscometers. Trans. Soc. Rheol. 21:535–565, 1977.
Finger, E. B., K. Purl, R. Alon, M. B. Lawrence, U. H. von Andrian, and T. A. Springer. Adhesion through L–selectin requires a threshold hydrodynamic shear. Nature (London) 379:266–269, 1996.
Fuhlbrigge, R. C., R. Alon, K. D. Puri, J. B. Lowe, and T. A. Springer. Sialylated, fucosylated ligands for L–selectin expressed on leukocytes mediate tethering and rolling adhesions in physiologic flow conditions. J. Cell Biol. 135:837–848, 1996.
Fung, Y. C. Biomechanics. Mechanical Properties of Living Tissues. New York: Springer, 1993.
Goboury, J. P., and P. Kubes. Reductions in physiologic shear rates lead to CD11/CD18–dependent, selectin–independent leukocyte rolling in vivo. Blood 83:345–350, 1994.
Goldsmith, H. L. Red cell motions and wall interactions in tube flow. Fed. Proc. 30:1578–1588, 1971.
Goldsmith, H. L., A. T. Quinn, G. Drury, C. Spanos, F. A. McIntosh, and S. I. Simon. Dynamics of neutrophil aggregation in couette flow revealed by videomicroscopy: Effect of shear rate on two–body Collision efficiency and doublet lifetime. Biophys. J. 81:2020–2034, 2001.
Gopalan, P. K., C. W. Smith, H. Lu, E. L. Berg, L. V. McIntire, and S. I. Simon. Neutrophil CD18–dependent arrest on ICAM–1 in shear flow can be activated through L–selectin. J. Immunol. 158:367–375, 1997.
Guyer, D. A., K. L. Moore, E. B. Lynam, C. M. G. Schammel, S. Rogelj, R. P. McEver, and L. A. Sklar. P–selectin glycoprotein ligand–1 (PSGL–1) is a ligand for L–selectin in neutrophil aggregation. Blood 88:2415–2421, 1996.
Hammerschmidt, D. E., P. R. Craddock, J. McCullough, R. S. Kronenberg, A. P. Dalmasso, and H. S. Jacob. Complement activation and pulmonary leukostasis during nylon fiber filtration leukapheresis. Blood 51:721–730, 1978.
Hentzen, E. R., S. Neelamegham, G. S. Kansas, J. A. Benanti, L. V. McIntire, C. W. Smith, and S. I. Simon. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule–1. Blood 95:911–920, 1999.
Jones, D. A., O. Abbassi, L. V. McIntire, R. P. McEver, and C. W. Smith. P–selectin mediates neutrophil rolling on histamine–stimulated endothelial cells. Biophys. J. 65:1560–1569, 1993.
Jung, U., K. E. Norman, K. Scharffetter–Kochanek, A. L. Beaudet, and K. Ley. Transit time of leukocytes rolling through venules controls cytokine–induced inflammatory cell recruitment in vivo. J. Clin. Invest. 102:1526–1533, 1998.
Kansas, G. S. Selectins and their ligands: Current concepts and controversies. Blood 88:3259–3287, 1996.
Kishimoto, T. K. The selectins. In: Structure, Function, and Regulation of Molecules Involved in Leukocyte Adhesion, edited by P. E. Lipsky, R. Rothlein, T. K. Kishimoto, R. B. Faanes, and C. W. Smith. New York: Springer, 1993, pp. 107–134.
Kuijpers, T. W., L. Koenderman, R. S. Weening, A. J. Verhoeven, and D. Roos. Continuous cell activation is necessary for stable interaction of complement receptor type 3 with its counter–structure in the aggregation response of human neutrophils. Eur. J. Immunol. 20:501–508, 1990.
Lawrence, M. B., G. S. Kansas, E. J. Kunkel, and K. Ley. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E). J. Cell Biol. 136:717–727, 1997.
Lawrence, M. B., and T. A. Springer. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell 65:859–873, 1991.
Lehr, H.–A., A. S. Weyrich, R. K. Saetzler, A. Jurek, K. E. Arfors, G. A. Zimmerman, S. M. Prescott, and T. M. McIntyre. Vitamin C blocks inflammatory platelet–activating factor mimetics created by cigarette smoking. J. Clin. Invest. 99:2358–2364, 1997.
Lu, H., C. M. Ballantyne, and C. W. Smith. LFA–1 (CD11a/CD18) triggers hydrogen peroxide production by canine neutrophils. J. Leukoc Biol. 68:73–80, 2000.
Lu, H., C. W. Smith, J. Perrard, D. Bullard, L. Tang, S. B. Shappell, M. L. Entman, A. L. Beaudet, and C. M. Ballantyne. LFA–1 is sufficient in mediating neutrophil emigration in Mac–1 deficient mice. J. Clin. Invest. 99:1340–1350, 1997.
Lupher, Jr., M. L., E. A. Harris, C. R. Beals, L. Sui, R. C. Liddington, and D. E. Staunton. Cellular activation of leukocyte function–associated antigen–1 and its affinity are regulated at the i domain allosteric site. J. Immunol. 167:1431–1439, 2001.
Lynam, E. B., S. I. Simon, Y. P. Rochon, and L. A. Sklar. Lipopolysaccharide enhances CD11b/CD18 function but inhibits neutrophil aggregation. Blood 83:3303–3311, 1994.
McDonagh, P. F., D. S. Wilson, H. Iwamura, C. W. Smith, S. K. Williams, and J. G. Copeland. CD18 Antibody treatment limits early myocardial reperfusion injury after initial leukocyte deposition. J. Surg. Res. 64:139–149, 1996.
Melder, R. J., J. Yuan, L. L. Munn, and R. K. Jain. Erythrocytes enhance lymphocyte rolling and arrest in vivo. Microvasc. Res. 59:316–322, 2000.
Merkel, R., P. Nassoy, A. Leung, K. Ritchie, and E. Evans. Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy (Comments). Nature (London) 397:50–53, 1999.
Mitchell, D. J., P. Li, P. H. Reinhardt, and P. Kubes. Importance of L–selectin–dependent leukocyte–leukocyte interactions in human whole blood. Blood 95:2954–2959, 2000.
Munn, L. L., R. J. Melder, and R. K. Jain. Role of erythrocytes in leukocyte–endothelial interactions: Mathematical model and experimental validation. Biophys. J. 71:466–478, 1996.
Neelamegham, S., A. D. Taylor, A. R. Burns, C. W. Smith, and S. I. Simon. Hydrodynamic shear shows distinct roles for LFA–1 and Mac–1 in neutrophil adhesion to intercellular adhesion molecule–1. Blood 92:1626–1638, 1998.
Neelamegham, S., A. D. Taylor, J. D. Hellums, M. Dembo, C. W. Smith, and S. I. Simon. Modeling the reversible kinetics of neutrophil aggregation under hydrodynamic shear. Biophys. J. 72:1527–1540, 1997.
Neelamegham, S., A. D. Taylor, H. Shankaran, C. W. Smith, and S. I. Simon. Shear and time–dependent changes in Mac–1, LFA–1, and ICAM–3 binding regulate neutrophil homotypic adhesion. J. Immunol. 164:3798–3805, 2000.
Okuyama, M., J. Kambayashi, M. Sakon, and M. Monden. LFA–1/ICAM–3 mediates neutrophil homotypic aggregation under fluid shear stress. J. Cell. Biochem. 60:550–559, 1996.
Ramachandran, V., T. Yago, T. K. Epperson, M. M. Kobzdej, M. U. Nollert, R. D. Cummings, C. Zhu, and R. P. McEver. Dimerization of a selectin and its ligand stabilizes cell rolling and enhances tether strength in shear flow. Proc. Natl. Acad. Sci. U.S.A. 98:10166–10171, 2001.
Rochon, Y. P., and M. M. Frojmovic. Dynamics of human neutrophil aggregation evaluated by flow cytometry. J. Leukoc Biol. 50:434–443, 1991.
Schmid–Schonbein, G. W., S. Usami, R. Skalak, and S. Chien. The interaction of leukocytes and erythrocytes in capillary and post–capillary vessels. Microvasc. Res. 19:45–70, 1980.
Schmidtke, D. W., and S. L. Diamond. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P–selectin under physiological flow. J. Cell Biol. 149:719–730, 2000.
Shankaran, H., and S. Neelamegham. Nonlinear flow affects hydrodynamic forces and neutrophil adhesion rates in coneplate viscometers. Biophys. J. 80:2631–2648, 2001.
Sigal, A., A. D. Bleijs, V. Grabovsky, S. J. van Vliet, O. Dwir, C. G. Figdor, Y. van Kooyk, and R. Alon. The LFA–1 integrin supports rolling adhesions on ICAM–1 under physiological shear flow in a permissive cellular environment. J. Immunol. 165:442–452, 2000.
Simon, S. I., A. R. Burns, A. D. Taylor, P. K. Gopalan, E. B. Lynam, L. A. Sklar, and C. W. Smith. L–Selectin (CD62L) crosslinking signals neutrophil adhesive functions via the Mac–1 (CD11b/CD18) β 2–integrin. J. Immunol. 155:1502–1514, 1995.
Simon, S. I., J. D. Chambers, and L. A. Sklar. Flow cytometric analysis and modeling of cell–cell adhesive interactions: The neutrophil as a model. J. Cell Biol. 111:2747–2756, 1990.
Simon, S. I., Y. Hu, D. Vestweber, and C. W. Smith. Neutrophil tethering on E–selectin activates β2 integrin binding to ICAM–1 through a mitrogen–activated protein kinase signal transduction pathway. J. Immunol. 164:4348–4358, 2000.
Simon, S. I., S. Neelamegham, A. Taylor, and C. W. Smith. The multistep process of homotypic neutrophil aggregation: A review of the molecules and effects of hydrodynamics. Cell Adhes Commun. 6:263–276, 1998.
Simon, S. I., Y. P. Rochon, E. B. Lynam, C. W. Smith, D. C. Anderson, and L. A. Sklar. Beta2–integrin and L–selectin are obligatory receptors in neutrophil aggregation. Blood 82:1097–1106, 1993.
Smith, C. W. Endothelial adhesion molecules and their role in inflammation. Can. J. Physiol. Pharmacol. 71:76–87, 1993.
Smith, C. W., A. R. Burns, and S. I. Simon. 1998. Cooperative signaling between leukocytes and endothelium mediating firm attachment. In: Vascular Adhesion Molecules and Inflammation, edited by J. D. Pearson. Basel: Birkhauser, 1998, pp. 39–64.
Smith, M. J., E. L. Berg, and M. B. Lawrence. A direct comparison of selectin–mediated transient, adhesive events using high temporal resolution. Biophys. J. 77:3371–3383, 1999.
Smolen, J. E., T. K. Petersen, C. Koch, S. J. O'Keefe, W. A. Hanlon, S. Seo, D. Pearson, M. C. Fossett, and S. I. Simon. L–selectin signaling of neutrophil adhesion and degranulation involves p38 MAP kinase. J. Biol. Chem. 275:15876–15884, 2000.
Smoluchowski, M. V. Versuch einer mathematischen theorie der koagulationskinetik kolloider losungen. Z. Phys. Chem. (Leipzig) 92:129–168, 1917.
Springer, T. A. Adhesion receptors of the immune system. Nature (London) 346:425–434, 1990.
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76:301–314, 1994.
Tandon, P., and S. L. Diamond. Kinetics of beta2–integrin and L–selectin bonding during neutrophil aggregation in shear flow. Biophys. J. 75:3163–3178, 1998.
Taylor, A. D., S. Neelamegham, J. D. Hellums, C. W. Smith, and S. I. Simon. Molecular dynamics of of the transition from L–selectin–to β2–integrin–dependent neutrophil adhesion under defined hydrodynamic shear. Biophys. J. 71:3488–3500, 1996.
Tees, D. F. J., O. Coenen, and H. L. Goldsmith. Interaction forces between red cells agglutinated by antibody. IV. Time and force dependence of break–up. Biophys. J. 65:1318–1334, 1993.
Tha, S. P., and H. L. Goldsmith. Interaction forces between red cells agglutinated by antibody. I. Theoretical. Biophys. J. 50:1109–1116, 1986.
Tsang, Y. T. M., S. Neelamegham, Y. Hu, E. L. Berg, A. R. Burns, C. W. Smith, and S. I. Simon. Synergy between L–selectin signaling and chemotactic activation during neutrophil adhesion and transmigration. J. Immunol. 159:4566–4577, 1997.
van de Ven, T. G. M. Colloidal Hydrodynamics. San Diego: Academic, 1989.
van de Ven, T. G. M., and S. G. Mason. The microrheology of colloidal dispersions. VII. Orthokinetic doublet formation of spheres. Colloid Polym. Sci. 255:468–479, 1977.
Vestweber, D., and J. E. Blanks. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 79:181–213, 1999.
von Andrian, U. H., E. M. Berger, J. D. Chambers, L. Ramezani, H. Ochs, J. M. Harlan, J. C. Paulson, A. Etzioni, and K.–E. Arfors. In vivo behavior of neutrophils from two patients with distinct inherited leukocyte adhesion deficiency syndromes. J. Clin. Invest. 91:2893–2897, 1993.
von Andrian, U. H., J. D. Chambers, L. M. McEvoy, R. F. Bargatze, K.–E. Arfors, and E. C. Butcher. Two step model of leukocyte–endothelial cell interaction in inflammation: Distinct roles for LECAM–1 and the leukocyte beta2–integrins in vivo. Proc. Natl. Acad. Sci. U.S.A. 88:7538–7542, 1991.
von Andrian, U. H., P. Hansell, J. D. Chambers, E. M. Berger, I. T. Filho, E. C. Butcher, and K.–E. Arfors. L–selectin function is required for beta2–integrin–mediated neutrophil adhesion at physiologic shear rates in vivo. Am. J. Physiol. 263:H1034–H1044, 1992.
Waddell, T. K., L. Kialkow, C. K. Chan, T. K. Kishimoto, and G. P. Downey. Signaling functions of L–selectin. J. Biol. Chem. 270:15403–15410, 1995.
Walcheck, B., K. L. Moore, R. P. McEver, and T. K. Kishimoto. Neutrophil–neutrophil interactions under hydrodynamic shear stress involve L–selectin and PSGL–1. A mechanism that amplifies initial leukocyte accumulation on P–selectin in vitro. J. Clin. Invest. 98:1081–1087, 1996.
Xia, Z., and M. M. Frojmovic. Aggregation efficiency of activated normal or fixed platelets in a simple shear field: Effect of shear and fibrinogen occupancy. Biophys. J. 66:2190–2201, 1994.
Youker, K. A., J. Beirne, J. Lee, L. H. Michael, C. W. Smith, and M. L. Entman. Time–dependent loss of Mac–1 from in–filtrating neutrophils in the reperfused myocardium. J. Immunol. 164:2752–2758, 2000.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simon, S.I., Goldsmith, H.L. Leukocyte Adhesion Dynamics in Shear Flow. Annals of Biomedical Engineering 30, 315–332 (2002). https://doi.org/10.1114/1.1467677
Issue Date:
DOI: https://doi.org/10.1114/1.1467677